US Pharm. 2021;46(1):HS-1-HS-5.
ABSTRACT: Multiple sclerosis (MS) is an autoimmune disease that attacks the central nervous system, affecting nearly 1 million people in the United States. Recently, two new sphingosine-1 phosphate (SIP1) receptor modulators, siponimod and ozanimod, were FDA-approved for the treatment of clinically isolated syndrome, relapsing-remitting MS (RRMS), and secondary progressive MS (SPMS). In clinical trials, ozanimod was found to significantly reduce the annualized relapse rate in patients with RRMS compared with interferon. Siponimod was compared against placebo in patients with SPMS and showed a statistically significant decrease in disability progression at 3 months. A review of newly approved SIP1 therapy illustrates further advancements and therapy options for decreased relapse rate and progression in adult MS patients.
Multiple sclerosis (MS) is an autoimmune disease that attacks the central nervous system (CNS) by way of inflammation, demyelination, astroglial scarring, and neuronal loss. There is a distinctive accumulation of macrophages and lymphocytes most likely causing the inflammation.1,2 Nearly 1 million people in the United States have MS, with an annual average growth rate of 2.3% from 2010 to 2017.3 MS is more commonly found in women than men, with onset between ages 20 and 40 years.2,3 MS is considered to have variable symptoms. The disease progression is characterized by progressive neurologic and physical disability, usually marked by acute attacks.1 MS can be divided into the following four basic clinical disease courses:
Clinically isolated syndrome (CIS) is the first episode of neurologic symptoms that lasts at least 24 hours and is caused by inflammation or demyelination in the CNS. A CIS event does not necessarily mean a patient has MS, as this event may or may not lead to MS. CIS with MRI-detected brain lesions similar to those seen in MS have a high likelihood of progression to MS.1,2
Relapsing-remitting MS (RRMS) has clearly defined attacks that can evolve over days or weeks, followed by periods of partial or complete recovery in the ensuing weeks to months of remission. Active RRMS has relapses and/or new MRI activity. Attacks that only have a partial recovery lead to an increase in disability during remission. RRMS accounts for approximately 85% of MS cases.1,2
Secondary progressive MS (SPMS) always begins as RRMS but eventually transitions to SPMS due to progressive and worsening neurologic function over time. As with RRMS, active SPMS will have relapses or new activity on MRI. Progressive SPMS will also have increased disability (with or without relapses) or new MRI lesions. The risk of developing SPMS from RRMS increases approximately 2% each year.1,2
Primary progressive MS (PPMS) is characterized by worsening neurologic function from the onset of symptoms but with no early relapses or remissions. In time, active PPMS can have relapses or progression with or without relapses or MRI activity.1,2
While there is still no curative treatment available for MS, in the last 20 years multiple options of disease-modifying therapies (DMTs) have been introduced for patients and their providers to choose from based on factors such as cost, monitoring, and side effects.4 In 2018, the American Academy of Neurology published a practice guideline highlighting DMT use in adults with MS. This guidance focuses on starting, modifying, and stopping DMTs. When starting therapy for RRMS, DMTs have been classified based upon their efficacy compared with placebo and against other DMTs, specifically interferon beta-1a and -1b.5 With multiple medications showing superior efficacy, treatment decisions should be based upon individual preference to medications and side-effect profile. Since the publication of these guidelines, ozanimod (Zeposia) and siponimod (Mayzent) have received FDA approval. With their recent approvals, we will review the sphingosine-1 phosphate (SIP1) receptor modulators, including fingolimod (Gilenya).
Mechanism of Action
SIP1 modulation leads to altered responses involved in immunity, heart rate, smooth muscle, and endothelial barrier function.3 SIP1 has five different subtypes labeled 1 to 5. In MS, the clinical effect of SIP1 modulation results from binding subtype 1. It is hypothesized that binding to subtype 1 increases lymphocyte sequestration into lymph nodes, leading to decreased circulating lymphocytes and presumed reduction of lymphocyte migration into the CNS. This appears to be a dose-dependent relationship.6,7 Ozanimod and siponimod are selective to SIP1 subtypes 1 and 5, while fingolimod binds to subtypes 1, 3, 4, and 5. Binding to additional subtypes may lead to adverse events.
Clinical Trials
Ozanimod and siponimod are currently FDA-approved and have established clinical efficacy for the treatment for adults with CIS, RRMS, and active forms of SPMS.8,9 Fingolimod is currently FDA-approved for (and has established clinical efficacy for the treatment of) CIS, RRMS, and active SPMS in patients aged 10 years and older.10
Ozanimod
A 24-month, multicenter, double-blind, double-dummy phase III (RADIANCE) trial evaluated the safety and efficacy of ozanimod 0.92 mg and 0.45 mg against interferon beta-1a in 1,320 patients with a relapsing form of MS.11 Baseline characteristics were similar between the three groups, with most study participants being enrolled in Eastern Europe. The primary endpoint was annualized relapse rate (ARR), the average number of relapses the study group has in a single year over a 24-month period. An adjusted ARR of 0.17 was observed in the ozanimod 0.92-mg group and 0.22 in the 0.46-mg group, compared with 0.28 in the interferon group (P <.001 in the 0.92-mg group vs. interferon). An approximately 40% relative risk reduction was seen for ozanimod 0.92-mg dosing. Secondary endpoints, including the number of new or enlarging T2 lesions per scan over 24 months and the number of gadolinium-enhancing lesions at 24 months, also statistically significantly favored ozanimod at both 0.46-mg and 0.92-mg dosing, with relative risk reductions of approximately 50%.11
This evidence was supported in a second 12-month, randomized, double-blind, double-dummy phase III (SUNBEAM) trial. In similar design, 1,346 patients were enrolled comparing ozanimod 0.46 mg and 0.92 mg to interferon beta-1a. An ARR of 0.18 observed in the ozanimod 0.92-mg group, 0.24 in the ozanimod 0.46-mg group, and 0.35 in the interferon beta-1a group further supported the use of ozanimod (P <.001 in the 0.92-mg group vs. interferon).12 Secondary outcomes also statistically favored the higher dose of ozanimod in new or enlarging T2 lesions and gadolinium-enhancing lesions over a 12-month period, leading the FDA to approve dosing titration to a dose of 0.92 mg once daily.12
A combined analysis of the RADIANCE and SUNBEAM trials showed a 18% reduction in disease progression compared with interferon beta-1a (95% CI, 0.062-0.104).11 Unfortunately, the combined trial numbers were underpowered to determine significance, and a low baseline disability score rendered it difficult to determine if the reduction in disease progression was a true reduction or an artifact of decreased disease relapse.
Siponimod
Siponimod’s efficacy and safety were evaluated based on an event- and exposure-driven, multicenter, double-blind, phase III (EXPAND) trial. Patients were enrolled based on a clinical diagnosis of SPMS. Two trial groups were randomized; 1,110 patients received 2-mg doses of siponimod, and 546 patients received a placebo. Baseline characteristics were similar between the two groups. The primary outcome was the time to a 3-month confirmed disability progression, which was defined as a 1-point increase in an expanded disability status scale (EDSS) or a 0.5 increase if the baseline EDSS score was greater than 5.5. The EDSS scale is a 0-to-10 scale, with zero indicating no disability, 3 to 4.5 scoring moderate disability but can still walk, and 10 representing death.13 Siponimod showed a statistically significant decrease in disability progression at 3 months, with 288/1096 (26%) with confirmed disability on 2 mg, while 173/545 (32%) confirmed disability while on placebo (hazard ratio [HR] 0.79; 95% CI, 0.65-0.95).14
Key secondary endpoints of the EXPAND trial included time to a 20% confirmed worsening from baseline and change from baseline T2 lesion volume. Siponimod was statistically favored in outcome change T2 lesion volume at 12 and 24 months. There was no statistical difference compared with placebo in time to 20% confirmed worsening (HR, 0.94; 95% CI, 0.80-1.10).14 Although not a focus of the trial, gadolinium-enhancing lesions and ARR were also compared. Siponimod outperformed placebo, but it is hard to compare directly to ozanimod due to the absence of an active comparator.
Fingolimod
Fingolimod has the most efficacy data supporting its use due to FDA approval in 2010, with pediatric use approved in 2018. Adult clinical trials for comparison versus ozanimod and siponimod will be highlighted. Fingolimod’s safety and efficacy were first proven in a 2-year phase III (FREEDOMS) trial against placebo. This was followed by two 12-month randomized, controlled trials against the active comparators interferon beta-1a and glatiramer acetate.
In the double-blind, double-dummy (TRANSFORMS) trial, 1,153 patients were randomized to treatment of fingolimod 1.25 mg, fingolimod 0.5 mg, or intramuscular interferon beta-1a. Baseline characteristics were well balanced between groups. The primary outcome, ARR, favored fingolimod with an ARR of 0.20 with fingolimod 1.25 mg, 0.16 with fingolimod 0.5 mg, and 0.33 with interferon beta-1a (P <.001 at both fingolimod doses).15 Secondary outcomes included new or enlarged lesions on T2-weighted images and gadolinium-enhancing lesions. Fingolimod was statistically favored in secondary outcomes at both the 1.25-mg and 0.5-mg dosing. More patients had adverse effects with fingolimod 1.25 mg and, therefore, were more likely to stop the study medication due to adverse events.15
Published in August 2020, a phase IIIb multicenter, randomized, rater-blinded, double-blinded (ASSESS) trial compared a total of 1,064 patients who received either fingolimod 0.5 mg, fingolimod 0.25 mg, or glatiramer acetate 20 mg. The primary outcome of ARR was studied up to 12 months, with an ARR of 0.15 with fingolimod 0.5 mg, 0.22 with fingolimod 0.25 mg, and 0.26 with glatiramer acetate (P = .01 for fingolimod 0.5 mg).16 Similar to the previous studies, secondary outcomes included new or enlarging T2 lesions at 12 months and gadolinium-enhancing lesions at 12 months. Both fingolimod 0.5 mg and 0.25 mg showed statistically significant decreases in secondary outcomes, but only fingolimod 0.5 mg showed a statistical difference in primary outcome ARR.16 These results demonstrated that fingolimod 0.5 mg was superior to glatiramer acetate.
Adverse Reaction and Safety Concerns
As of March 2020, ozanimod is the third oral SIP1 receptor modulator to come to market for MS, after fingolimod in 2010 and siponimod in 2019. Due to the risk of cardiac side effects, such as bradycardia and heart block, dose titrations are recommended for ozanimod and siponimod, with direct monitoring after the first dose of siponimod and fingolimod.17-19 Additionally, siponimod requires CYP2C9 genotyping before initiation to determine appropriate dosing (TABLE 1).19 All of the SIP1 receptor modulators will interact with other medications that will also cause bradycardia or QT prolongation. However, ozanimod has less risk of this interaction than the other two.19-21
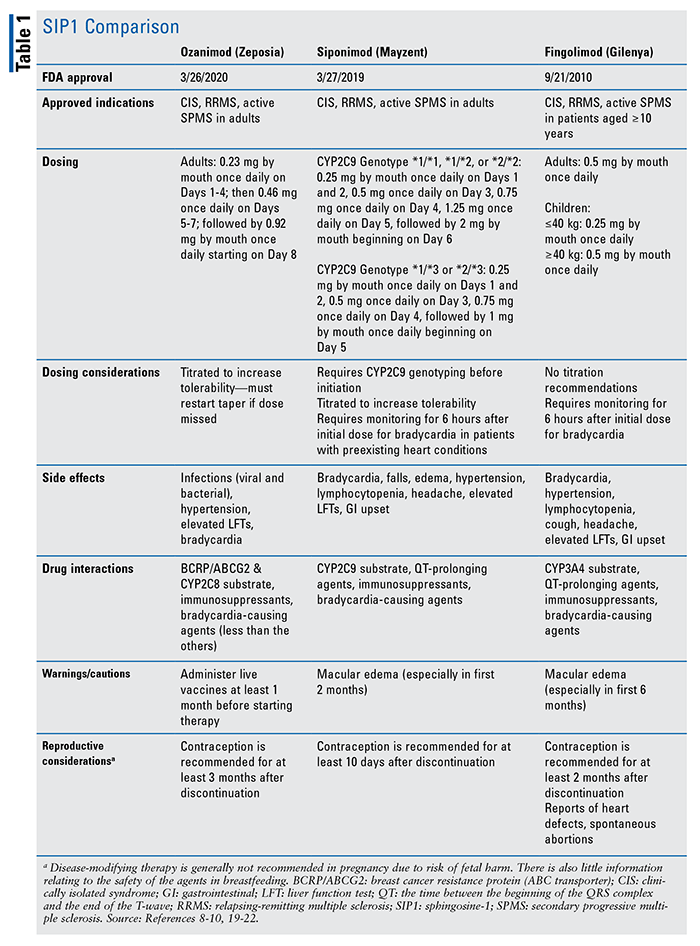
Fingolimod and siponimod are substrates of CYP3A4 and CYP2C9, respectively, and so it is important to check that the patient is not taking inducers or inhibitors of those enzymes.18,19,21 Fingolimod and siponimod carry the risk of macular edema for a short time after initiation of therapy. While it is still recommended to monitor for macular edema in ozanimod, it does not seem to carry the same risk.17,20 This highlights some of the prominent adverse effects of SIP1 receptor modulators. Prior to establishing treatment, however, all adverse effects, precautions, and contraindications should be reviewed for the individual patient.
Conclusion
The addition of ozanimod and siponimod to the SIP1 agonist class further provides support for their use in decreasing relapse rates in MS. It is currently unclear whether these agents modify disability progression over long periods of time, and this may be taken into consideration in a stepwise treatment approach to MS. With the other approved agents on the market for treatment of MS, the consideration to start an SIP1 agonist will likely include an individualized patient approach, including efficacy, safety, and cost considerations.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. National Multiple Sclerosis Society. Types of MS. www.nationalmssociety.org/What-is-MS/Types-of-MS. Accessed November 5, 2020.
2. Kasper DL, Channing WE, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw Hill Education; 2015.
3. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
4. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96-120.
5. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777-788.
6. Subei AM, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis. CNS Drugs. 2015;29(7):565-575.
7. Harris S, Tran JQ, Southworth H, et al. Effect of the sphingosine-1-phosphate receptor modulator ozanimod on leukocyte subtypes in relapsing MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e839.
8. Zeposia [package insert]. Summit, NJ: Calgene Corporation; September 2020.
9. Mayzent [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; July 2020.
10. Gilenya [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; July 2020.
11. Cohen JA, Comi G, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18(11):1021-1033.
12. Comi G, Kappos L, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18(11):1009-1020.
13. Wiendl H, Meuth SG. Pharmacological approaches to delaying disability progression in patients with multiple sclerosis. Drugs. 2015;75(9):947-977.
14. Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263-1273.
15. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402-415.
16. Cree BAC, Goldman MD, Corboy JR, et al. Efficacy and safety of 2 fingolimod doses vs glatiramer acetate for the treatment of patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA Neurol. [ePub August 24, 2020].
17. Ozanimod. Lexicomp Online [database online]. Hudson (OH): Wolters Kluwer Health; [updated 2020 Nov 4; cited 2020 Oct 24]. https://online.lexi.com.
18. Fingolimod. Lexicomp Online [database online]. Hudson (OH): Wolters Kluwer Health; [updated 2020 Nov 4; cited 2020 Oct 24]. Available from: https://online.lexi.com.
19. Siponimod. Clinical Pharmacology powered by ClinicalKey [data
base online]. Tampa (FL): Elsevier; [updated 2020; cited 2020 Oct 24]. www.clinicalpharmacology.com.
20. Ozanimod. Clinical Pharmacology powered by ClinicalKey [database online]. Tampa (FL): Elsevier; [updated 2020; cited 2020 Oct 24]. www.clinicalpharmacology.com.
21. Fingolimod. Clinical Pharmacology powered by ClinicalKey [database online]. Tampa (FL): Elsevier; [updated 2020; cited 2020 Oct 24]. www.clinicalpharmacology.com.
22. Fingolimod (Briggs Drugs in Pregnancy and Lactation). Lexicomp Online [database online]. Hudson (OH): Wolters Kluwer Health; [updated 2017 Nov 15; cited 2020 Oct 24.] https://online.lexi.com.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






