US Pharm.
2008;33(2):HS-3-HS-15
Currently,
sepsis--a systemic inflammatory response syndrome initiated secondary to an
infection--is the tenth leading cause of death in the United States.1
Approximately 750,000 patients develop sepsis each year.2,3
Sepsis-related mortality ranges from 30% to 50%, with advancing age increasing
the risk.4,5 Two factors that contribute to a positive outcome in
the treatment of sepsis are the timely diagnosis and identification of the
offending pathogen. The four cornerstones of the therapeutic management of
sepsis are hemodynamic monitoring, volume resuscitation, inotropic therapy,
and red blood cell transfusions. Despite therapeutic advances, sepsis-related
morbidity and mortality continue to increase.3
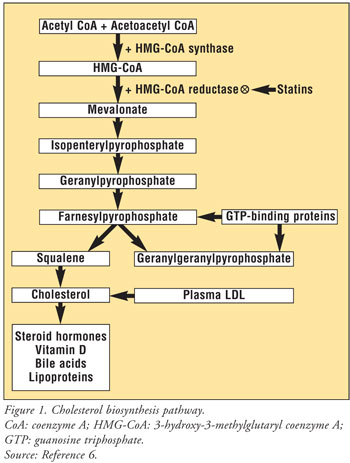
Hydroxymethylglutaryl coenzyme
A reductase inhibitors (statins) are an integral part of the medication
armamentarium for treating hyperlipidemia and reducing the risk of coronary
artery disease. Their beneficial effects are due to statins' ability to
inhibit the rate-limiting step of cholesterol biosynthesis, thereby decreasing
the amount of circulating cholesterol in the body (FIGURE 1).6
These well-documented benefits are termed lipid-dependent effects. It
has recently been discovered that statins possess effects beyond their
lipid-lowering properties; these are termed lipid-independent or
pleiotropic effects. Pleiotropic effects include anti-inflammatory,
immunomodulatory, antioxidant, antithrombotic, and endothelium-stabilizing
properties. Patients with sepsis syndrome experience dysfunction in all of
these areas; therefore, statins theoretically would benefit them.6†
The overall clinical impact of statins for treating sepsis is not fully
delineated, however.
Rationale for Sepsis Improvement
After Statin Use
Cytokines
6,7:
Sepsis activates an inflammatory cascade in which large amounts of cytokines
are released into the body. Macrophages and endothelial cells are then
hyperactivated by the unusually large quantity of circulating cytokines. The
activation of macrophages and endothelial cells results in the release of more
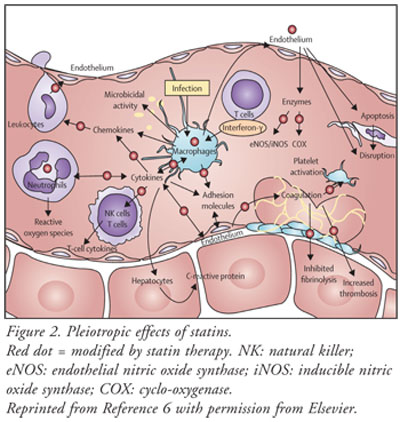
cytokines, exacerbating the inflammatory response. Statins have the ability to
alter the expression of various cytokines (FIGURE 2). This alteration
may lead to a reduction in the release of cytokines, thus breaking the chain
between cytokine release and activation of macrophages and endothelial cells.
These events potentially could culminate in the blunting of the inflammatory
component associated with sepsis syndrome.
C-Reactive Protein6,7
:
C-reactive protein (CRP) is a nonspecific marker for inflammation in the body.
Increased CRP levels have been associated with deleterious clinical outcomes.
CRP, which is produced by the liver, is driven by the release of cytokines. As
previously mentioned, sepsis precipitates an augmented chain of events that
ultimately results in the release of a large amount of cytokines. CRP assists
with endothelial-cell interaction, aids in the activation of complement, and
induces tissue-factor expression. These actions work in concert to promote the
formation of thrombi. Statins have the ability to deter cytokine release, thus
leading to decreased amounts available to the liver for producing CRP. In
theory, this action would reduce systemic inflammation and improve clinical
outcomes in the septic patient.
Chemokines6,7:
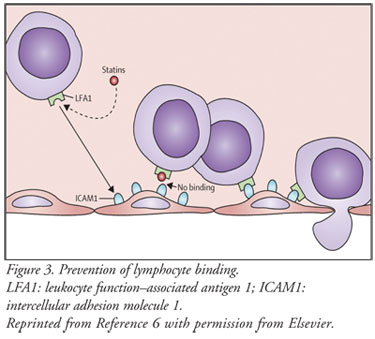
Chemokines attract
leukocytes to sites of infection or tissue damage. Statins have been shown to
bind directly to the lymphocyte's cell surface, which prevents binding to the
counterreceptor on the endothelial surface (FIGURE 3). Statins also
reduce the production of chemoattractants. These actions result in a net
decrease of inflammatory cells in the body. Statins also exert favorable
effects on T lymphocytes. The Th-1 subclass of T lymphocytes promotes
inflammation, which is inhibited by statins; the Th-2 subclass promotes
anti-inflammation, which is expressed by statins (FIGURE 2). Therefore,
statins are able to alter T lymphocytes, thereby halting an inflammatory
effect and inducing a net anti-inflammatory effect.
Coagulation6,7
:
Sepsis-induced inflammation causes the downregulation of thrombomodulin and
the upregulation of tissue factor on the surface of the endothelium. This
shift results in endothelial dysfunction and favors thrombosis. Statins
increase thrombomodulin on the endothelial surface and reduce tissue-factor
expression in endothelial cells. This action has the potential to reduce the
generation of thrombin. The increased amount of thrombomodulin on the surface
of the endothelium allows it to be readily available to bind with thrombin.
The net result of this binding is the activation of protein C. This activation
will result in the initiation of the intrinsic anticoagulant cascade.
Therefore, statins have the theoretical ability to blunt or reverse the
procoagulant state that exists in sepsis syndrome (FIGURE 2).
Enzymes6,7:
Sepsis causes an
increase in inducible nitric oxide (NO) synthase (NOS), which leads to the
overproduction of NO. This overproduction leads to excessive vasodilatation,
loss of systemic vascular resistance, and vascular leak. Statins modulate NOS
activity and, consequently, NO levels by reducing inducible NOS expression and
by maintaining or increasing endothelial constitutive NOS production. This
reduction in the inducible NOS/endothelial constitutive NOS ratio may be
clinically important since unselected NOS blockade is associated with
increased mortality (FIGURE 2).
Antioxidant Effects
6,7:
Reactive oxygen levels are increased in patients with sepsis. It has been
reported that oxidative stress is an important factor associated with
morbidity and mortality in patients with sepsis and multisystem organ failure.
Statins inhibit phorbol myristate acetateñinduced oxygen radical production in
monocytes through NADPH oxidase inactivation. Activation of this enzyme is
largely responsible for the production of reactive oxygen species in sepsis (
FIGURE 2).
Studies Addressing Statin
Therapy and Sepsis
Liappis et al
conducted a single-center, retrospective, cohort trial to compare mortality
and clinical findings in patients taking statins with those of patients not
receiving statins at the time of their bacteremic episode.8 Of the
388 subjects, 35 were taking a statin; the rest (n = 353) were not. The
authors found a significant reduction in overall hospital mortality rates
among patients taking statins, with a mortality rate of 6% in the statin group
and a rate of 28% in the nonstatin group. Mortality rates that could be
attributed to the infection were lower in the statin group (3%) than in the
nonstatin group (20%). The authors concluded that statins might prove
beneficial in the treatment of infections, but that prospective studies were
needed to validate this potential benefit. This trial had several weaknesses,
including a small number of subjects in the statin group, unbalanced baseline
characteristics, and a questionable study design.
The association between statin
administration and mortality in bacteremic patients requiring hospital
admission was assessed by Kruger et al in a single-center, retrospective,
cohort trial.9 This study involved 438 patients, 66 of whom were
receiving statin therapy upon admission (the remaining 372 were not). Compared
with the group that did not receive statins, the statin group had a
significantly lower incidence of hospital mortality (10.6% versus 23.1%) and
bacteremia-related mortality (6.1% versus 18.3%). There also was a reduction
in all-cause hospital mortality (1.8% versus 23.1%) and in death related to
bacteremia (1.8 versus 18.3%), which was more pronounced in patients who
continued to receive statin therapy after the diagnosis of bacteremia. The
authors concluded that statin administration provided a significant survival
benefit and recommended that more trials be conducted to evaluate statin
utilization in critically ill patients. Two weaknesses of this trial were the
small number of patients in the statin group and the inability to extrapolate
to a larger patient population.
Fernandez et al conducted a
single-center, retrospective, cohort trial of 438 subjects to assess the
impact of previous statin therapy on hospital mortality and determine whether
the impact is due to a protective effect against ICU-acquired infections.
10 Prior to ICU admission, 38 subjects were receiving statin therapy and
the remaining 400 were not. This trial found no difference with respect to
ICU-acquired infections between subjects who were taking statins preadmission
and those who were not receiving statin therapy. Paradoxically, there was a
higher rate of mortality in the statin group. The authors concluded that the
higher occurrence of mortality in the statin group might have been related to
the greater severity of illness in that group. The authors also suggested the
need for prospective, randomized trials in order to validate their findings.
Weaknesses of this trial were generalized inclusion criteria, no reason listed
for ICU admission, and differences in baseline characteristics between the two
groups that may have led to skewed results.
A single-center, prospective,
observational, cohort study undertaken by Almog et al evaluated whether
patients treated with statins developed severe sepsis less frequently and
whether this presumed protective effect might reduce the rate of admission to
the ICU.11 The study included 361 subjects, 82 of whom were
receiving statin therapy prior to hospital admission; the rest (n = 279) were
not receiving statin therapy. Severe sepsis developed in 2.4% of subjects
receiving prior statin therapy and in 19% of those not receiving it--a
statistical and clinical difference between the two groups. The subjects
receiving statin therapy also had a significantly reduced incidence of ICU
admission (3.7%) compared with the nonstatin group (12.2%). The investigators
found that therapy with statins for at least one month before the onset of an
acute bacterial infection is probably associated with a reduced rate of severe
sepsis and ICU admission. They reiterated a need for future prospective,
controlled trials to verify their results and to determine the clinical
significance of statins as a preventative approach to sepsis. Weaknesses of
this trial included unbalanced baseline characteristics, a relatively smaller
statin group, and the study design.
A population-based,
retrospective, cohort analysis was conducted by Thomsen et al to investigate
statin use and mortality due to bacteremia.12 This trial involved
5,353 subjects, 176 who had received statin therapy and 5,177 who had not. Up
to 30 days, mortality between the two groups was similar. However, between
days 31 and 180, the statin group experienced a significantly lower incidence
of mortality than the non-statin group. These results suggested that statins
may not possess short-term benefits in the treatment of bacteremia, but may
cause a reduction in long-term mortality. The researchers concluded that, due
to the trial's weaknesses, the benefits of statin therapy for bacteremia were
difficult to interpret. Two weaknesses were the relatively small size of the
statin group and the study design (observational).
Hackam et al engaged in a
large, population-based, retrospective, cohort study of 69,168 patients that
investigated the use of statins and their effect on sepsis.13 The
statin group and the nonstatin group each comprised 34,584 patients. The
authors found that the rate of sepsis was significantly lower in patients
receiving statin therapy prior to hospital admission compared with patients
not receiving statin therapy prior to admission. The design of this study was
considered a weakness due to the possibility that confounding factors had been
introduced.
A small, single-center,
retrospective, cohort trial of 53 subjects was carried out by Martin et al.
14 Sixteen subjects were receiving statin therapy and 37 controls were
not receiving statin therapy prior to admission. The trial investigated
whether the use of statins is associated with a reduced rate of severe sepsis
in a population of patients with confirmed sepsis; it also aimed to further
characterize the effect of statins on the frequency of organ dysfunction in
patients with severe sepsis. The authors established that statins were
associated with a significantly lower instance of severe sepsis (56%, versus
86% for the nonstatin group). No difference in mortality was noted between the
two groups (38% in the statin group and 49% in the non-statin group), however.
The statin group had a significant reduction in the rate of cardiovascular
dysfunction compared with controls (38% versus 73%). The authors concluded
that statins may provide an interventional strategy in patients with severe
sepsis or septic shock. They suggested further investigation in the form of
randomized, prospective, placebo-controlled trials involving patients with
sepsis. Weaknesses of this trial were its small sample size, analysis of a
single center, and retrospective evaluation.
Yang et al conducted a
retrospective study involving patients with sepsis.15 The objective
was to define the effect of statins on 30-day mortality in an Asian population
with sepsis. Of the 454 patients studied, 104 received a statin at least 30
days before hospital admission and during the course of the septic event. The
other 350 patients were considered controls and did not receive statin therapy
prior to admission. There was no significant difference in 30-day
sepsis-related mortality between the two groups (19.2% versus 18.9%). The
investigators concluded that there were no marked beneficial or harmful
effects of statin therapy during sepsis in terms of 30-day survival rates of
bloodstream infections in this population. Weaknesses of this trial were
unbalanced baseline characteristics and restrictive inclusion criteria.
Summary
The pleiotropic
effects associated with statin therapy are well documented and are gaining
increased attention. Sepsis is associated with inflammatory processes,
coagulopathies, oxidative stress, and vascular dysfunction. These deleterious
effects theoretically could be modified by statin therapy, which leads to the
assumption that statins may have beneficial effects in patients diagnosed with
sepsis. However, due to weak methodology and conflicting results, current
trials are unable to provide a definitive answer to this interesting question.
The available literature does suggest a positive association between statin
therapy and improved patient outcomes, but this must be validated by large,
randomized, double-blind, controlled trials. Statins cannot presently be
recommended as a therapeutic modality in the treatment or prevention of
sepsis.
References
1. National Center for Health Statistics. Deathsñleading causes. Available at: www.cdc.gov/nchs/fastats/lcod.htm. Accessed July 2007.
2. Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence outcome and associated costs of care. Crit Care Med. 2001;29:1303-1310.
3. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546-1554.
4. Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29(suppl 7):S109-S116.
5. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138-150.
6. Terblanche M, Almog Y, Rosenson RS, et al. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis. 2007;7:358-368.
7. Ray KK, Cannon CP. The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol. 2005;46:1425-1433.
8. Liappis AP, Kan VL, Rochester CG, Simon GL. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352-1357.
9. Kruger P, Fitzsimmons K, Cook D, et al. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med. 2006;32:75-79.
10. Fernandez R, De Pedro VJ, Artigas A. Statin therapy prior to ICU admission: protection against infection or a severity marker? Intensive Care Med. 2006;32:160-164.
11. Almog Y, Shefer A, Novack V, et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110: 880-885.
12. Thomsen RW, Hundborg HH, Johnsen SP, et al. Statin use and mortality within 180 days after bacteremia: a population-based cohort study. Crit Care Med. 2006;34:1080-1086.
13. Hackam DG, Mamdani M, Li P, Redelmeier DA. Statins and sepsis in patients with cardiovascular disease: a population-based cohort analysis. Lancet. 2006;367:413-418.
14. Martin CP, Talbert RL, Burgess DS, Peters JI. Effectiveness of statins in reducing the rate of severe sepsis: a retrospective evaluation. Pharmacotherapy. 2007;27:20-26.
15. Yang KC, Chien JY, Tseng WK, et
al. Statins do not improve short-term survival in an oriental population with
sepsis. Am J Emerg Med. 2007;25:494-501.
To comment on this article,
contact editor@uspharmacist.com.





