US Pharm. 2022;47(5):39-42.
ABSTRACT: Clostridioides difficile infection (CDI) has become one of the most common healthcare-associated infections, with symptoms ranging in severity from mild or moderate diarrhea to severe or fulminant colitis. The burden of CDI, especially the first recurrence, remains a concern, and treatment challenge and rates of community-associated infection are rising. With growing evidence suggesting that fidaxomicin may reduce CDI recurrence, the recently published focused update to the IDSA/SHEA guidelines highlight the use of fidaxomicin as preferred therapy over oral vancomycin. In addition, the use of bezlotoxumab is discussed as an adjunct therapy for CDI recurrence.
Clostridioides difficile infection (CDI) is attributed to C difficile, a toxin-producing, spore-forming, gram-positive anaerobic bacteria that is transmitted by the fecal-oral route. CDI has become one of the most common healthcare-associated infections, with symptoms ranging in severity from mild or moderate diarrhea to severe or fulminant colitis.1 While commonly considered a healthcare-associated infection, rates of CDI identified in the community are on the rise. From 2011 to 2017, the cases of healthcare-associated CDI have declined, whereas community-associated CDI cases have increased to nearly those of healthcare-associated infections. In 2017, an estimated 462,000 cases of CDI, including 236,000 healthcare-associated and 226,000 community-associated cases, occurred in the United States, with an estimated incidence of 143.6 per 100,000 population.2 Recurrence of infection was also common, with nearly 70,000 first CDI recurrences. Approximately 20,500 deaths were also attributed to CDI. While the number of cases has declined over recent years, likely a result of antimicrobial stewardship and infection prevention strategies, the burden of CDI, especially first recurrence of CDI, remains a concern and treatment challenge.
Antimicrobial exposure is one of the most significant risk factors for the development of infection due to the disruption in normal intestinal flora.3-5 Clindamycin, fluoroquinolones, and cephalosporins have been associated with the greatest risk of CDI in both the community and healthcare settings.4 Other risk factors for healthcare-associated CDI include advanced age, hospitalization in the previous 2 months, and treatment with chemotherapy or acid-suppressive therapy, such as histamine H2 blockers or proton-pump inhibitors.6 The risk factors for community-associated infection are less understood and may include prior antibiotic use, cardiac disease, chronic kidney disease, and inflammatory bowel disease.7 Although advanced age is commonly encountered in healthcare-associated infection, community-dwelling patients diagnosed with CDI tend to be younger. Recurrence of CDI occurs in up to 25% of patients, making prevention of recurrence an important goal of therapy.8 Risk factors for recurrence include age older than 65 years, recent hospitalization, immunocompromised state, and previous CDI.9
Treatment of CDI consists of metronidazole, vancomycin, or fidaxomicin. The 2017 CDI treatment guidelines, developed by the Infectious Diseases Society of America (IDSA) and Society of Healthcare Epidemiology of America (SHEA), recommended vancomycin or fidaxomicin over metronidazole for initial CDI episodes.10 Metronidazole, along with oral vancomycin, was previously employed as a primary antibiotic for CDI; however, several studies demonstrated reduced rates of clinical cure, particularly in the setting of severe disease.11 Metronidazole is not recommended first line for treatment of CDI, though this medication may still be utilized for nonsevere disease if vancomycin and fidaxomicin are not available. Cost considerations and pharmacy availability may also limit accessibility of these preferred treatments.
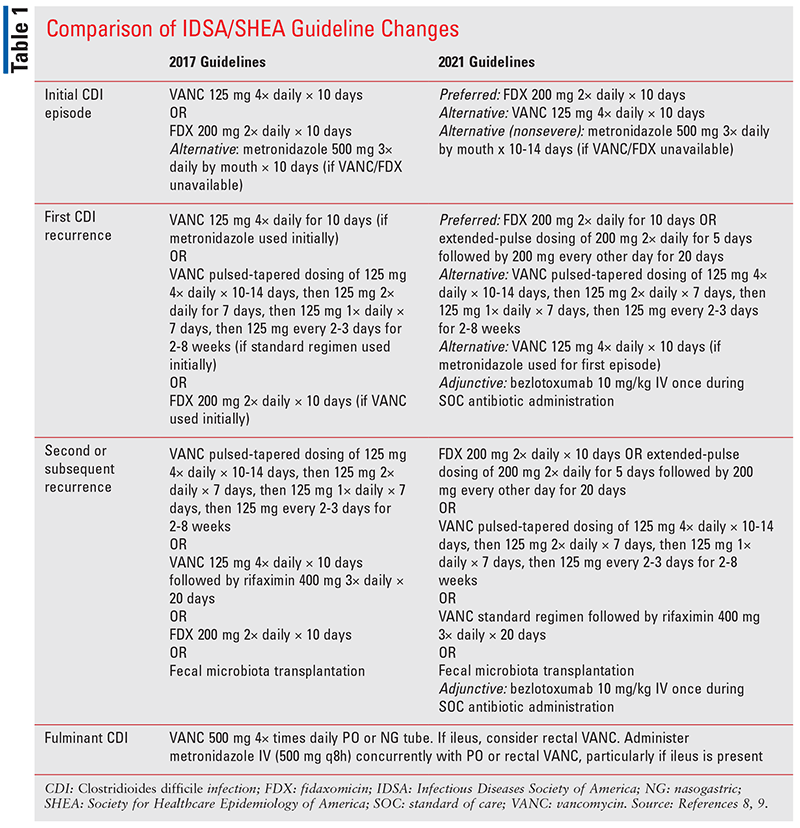
`With growing evidence suggesting that fidaxomicin may reduce CDI recurrence, the recently published focused update to the IDSA/SHEA guidelines highlights the use of fidaxomicin as preferred therapy over oral vancomycin.9 In addition, the use of bezlotoxumab is discussed as an adjunct therapy for recurrence. This review will focus primarily on the use of fidaxomicin and bezlotoxumab for the management of CDI. A detailed summary and comparison of CDI treatment recommendations from the 2017 and 2021 guidelines are presented in TABLE 1.
Fidaxomicin
Fidaxomicin, a macrocyclic antibacterial, was approved by the FDA in 2011 for the treatment of CDI.12 Fidaxomicin works primarily in the gastrointestinal tract with minimal systemic absorption and is excreted primarily in feces. Compared with oral vancomycin, fidaxomicin has a narrower spectrum of activity, primarily with action against Clostridia species, including C difficile, and lacks activity against many of the organisms that make up the normal microbiota of the intestines.13,14
As mentioned, a major update to the 2021 IDSA/SHEA guidelines is the recommendation to treat initial CDI episodes with fidaxomicin over oral vancomycin. Previous studies have demonstrated noninferiority of standard dosing of fidaxomicin (200 mg twice daily for 10 days) to vancomycin (125 mg four times daily for 10 days).15,16 Nearly 41% of patients in the prior study were treated as outpatients, with similar rates of clinical cure encountered among the outpatient subgroups. Additionally, patients treated with fidaxomicin in the outpatient subgroup had reduced rates of CDI recurrence.15 Though initial studies suggest reduced rate of recurrence with fidaxomicin compared with vancomycin, prior guidelines recommended either vancomycin or fidaxomicin for treatment of initial CDI episodes. Since the publication of the 2017 guidelines, additional studies have been published with similar results, finding reduced infection recurrence with fidaxomicin.17,18
The EXTEND trial was the first study to evaluate an extended-pulsed dose of fidaxomicin compared with standard dosing of vancomycin for CDI, where authors hypothesized that extended-pulsed dosing of fidaxomicin may result in sustained suppression of C difficile and, in turn, a sustained clinical response.18 Patients in the fidaxomicin group received a dose of 200 mg twice daily on Days 1 to 5 and 200 mg every other day on Days 7 to 25. The primary endpoint of this study was sustained clinical cure at 30 days following the end of treatment, defined as clinical response at test of cure and no CDI recurrence. Sustained clinical cure at 30 days was achieved in 70% of patients in the fidaxomicin group compared with 59% of patients in the vancomycin group (P = .030). Patients were followed up to Day 90, where patients in the fidaxomicin group had a significantly lower incidence of recurrence compared to vancomycin.
Previously considered suitable for first-line treatment of initial CDI episodes, vancomycin became the standard of care in many cases due in large part to the lower drug acquisition cost compared with fidaxomicin. A systematic review published in 2017 evaluated 27 economic evaluations for the treatment of CDI including metronidazole, vancomycin, and fidaxomicin.19 Fidaxomicin was found to be cost-effective in 58.3% of studies compared with metronidazole or vancomycin. Conversely, vancomycin was determined to be cost-effective versus fidaxomicin or metronidazole in only 18.5% of reviewed economic evaluations. Recently, additional studies have been performed to further evaluate the economic implications of fidaxomicin compared with vancomycin. Reveles et al reviewed costs associated with fidaxomicin or vancomycin as first-line therapy for C difficile–associated diarrhea.20 The total cost was similar between the two groups: $14,442 per patient in the fidaxomicin treatment group versus $14,179 for vancomycin. Although both treatments resulted in similar cost for the initial hospitalization, the higher drug costs seen with fidaxomicin were offset by lower recurrence rates, resulting in fewer rehospitalizations. A cost-effectiveness analysis of the EXTEND trial reviewed the extended-pulsed dose fidaxomicin versus vancomycin as first-line therapy for CDI.21 Fidaxomicin was associated with improvement in quality-adjusted life years. Additionally, drug acquisition costs were higher with extended-pulse fidaxomicin than for vancomycin, although lower total hospitalization costs and lower costs of managing adverse effects were realized, due in part to reduced CDI recurrence and adverse effects. Furthermore, a cost-effectiveness study published in 2020 found that initial treatment with fidaxomicin for nonsevere CDI and fidaxomicin for first recurrence of CDI were the most cost-effective options.22 This study supports the economic preference of fidaxomicin due to reduced risk of CDI recurrence and subsequent hospitalization.
Bezlotoxumab
The newest agent for CDI recurrence presented in the 2021 focused update to the guidelines is bezlotoxumab, a monoclonal antibody approved by the FDA in 2016.23 Bezlotoxumab acts via a novel mechanism to augment the therapeutic effects of antimicrobials; the primary mechanism of action is through binding of C difficile toxin B, neutralizing its effects. It is not an antibacterial agent and, therefore, is not utilized in the treatment of CDI in the absence of active therapy; however, it may be considered as an adjunct therapy in patients with CDI who are at high risk for recurrence. It is administered as a single 10 mg/kg IV dose infused over 60 minutes while the patient is receiving active treatment for CDI.
The safety and efficacy of bezlotoxumab were evaluated in two phase III, randomized, controlled trials: MODIFY I and MODIFY II.24 Patients with primary or recurrent CDI who were receiving standard-of-care antibiotics with metronidazole, vancomycin, or fidaxomicin were eligible for the study and were randomized to one of four groups: bezlotoxumab alone, actoxumab alone (MODIFY I only), bezlotoxumab + actoxumab, or placebo. Results of MODIFY I demonstrated a lack of efficacy of actoxumab (monoclonal antibody that binds to C difficile toxin A), therefore it was not evaluated in MODIFY II.
A total of 1,554 patients were randomized, 781 patients in the bezlotoxumab group and 773 patients in the placebo group. Baseline characteristics were similar between the two groups, with nearly 95% of patients on metronidazole or vancomycin and approximately 75% of patients having at least one risk factor for recurrence. Regardless of the antibacterial agent chosen for treatment, bezlotoxumab compared with placebo significantly reduced the incidence of recurrence during the 12-week follow- up period in both trials (MODIFY I: 17% vs. 28%, P <.001; MODIFY II: 16% vs 26%, P <.001). In a post hoc analysis of the MODIFY trials, no difference in recurrence rate was found in patients without any risk factors (defined as age ³65 years, compromised immunity, severe CDI, prior CDI episode, or infection with B1/NAP1/027 strains); however, in patients with one or more risk factors for recurrence, bezlotoxumab significantly decreased the rate of CDI recurrence.25 This finding was most pronounced among patients with three or more risk factors. These findings suggest that patients at higher risk for CDI recurrence derive the greatest benefit from bezlotoxumab administration.
In the MODIFY trials, the rate of adverse events reported during the 24 hours after the infusion occurred at similar rates between the two groups (10.3% vs. 7.6% in the bezlotoxumab and placebo groups, respectively).24 Of note, while the overall rate of serious adverse events was similar (29.4% vs. 32.7%), heart failure was reported in more patients in the bezlotoxumab compared with placebo (2.2% vs. 0.9%). Due to this increased rate of heart failure, the current prescribing information cautions against the use of bezlotoxumab in patients with a history of congestive heart failure.23 `
Conclusion
A review of available literature supports the recommendation of the 2021 IDSA/SHEA guidelines for the use of fidaxomicin as preferred therapy for initial episodes and first recurrence of CDI. Fidaxomicin has demonstrated efficacy, particularly decreased risk of recurrence, and improved safety compared to vancomycin. These benefits may relate to fidaxomicin’s narrower spectrum of activity, allowing for a preservation of intestinal flora. While fidaxomicin has a higher acquisition cost, the potential for prevention of recurrence of CDI may result in lower overall costs for patients.
Bezlotoxumab was added to the 2021 focused guideline update as an adjunct to prevent CDI recurrence. Studies suggest this monoclonal antibody provides the most benefit in patients with multiple risk factors for CDI recurrence. Given cost considerations, additional studies are needed to compare its use to fidaxomicin and to further delineate which patient groups may benefit the most.
The authors would like to thank Kassie Mann, PharmD candidate, Presbyterian College School of Pharmacy, for her work on this article.
REFERENCES
1. Czepiel J, Dróżdż M, Pituch H, et al. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38(7):1211-1221.
2. Guh AY, Mu Y, Winston LG, et al. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N Engl J Med. 2020;382(14):1320-1330.
3. Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhea: a systematic review. J Antimicrob Chemother. 2003;51(6):1339-1350.
4. Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013;68(9):1951-1961.
5. Johnson S, Gerding DN. Clostridium difficile--associated diarrhea. Clin Infect Dis. 1998;26(5):1027-1036.
6. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693-1703.
7. Fu Y, Luo Y, Grinspan AM. Epidemiology of community-acquired and recurrent Clostridioides difficile infection. Therap Adv Gastroenterol. 2021;14:17562848211016248.
8. Sheitoyan-Pesant C, Abou Chakra CN, Pépin J, et al. Clinical and Healthcare Burden of Multiple Recurrences of Clostridium difficile Infection. Clin Infect Dis. 2016;62(5):574-580.
9. Johnson S, Lavergne V, Skinner AM, et al. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin Infect Dis. 2021;73(5):e1029-e1044.
10. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48.
11. Okumura H, Fukushima A, Taieb V, Shoji S, English M. Fidaxomicin compared with vancomycin and metronidazole for the treatment of Clostridioides (Clostridium) difficile infection: a network meta-analysis. J Infect Chemother. 2020;26(1):43-50.
12. Dificid (fidaxomicin) package insert. Whitehouse Station, NJ. Merck & Co. Inc; 2021.
13. Cho JM, Pardi DS, Khanna S. Update on Treatment of Clostridioides difficile Infection. Mayo Clin Proc. 2020;95(4):758-769.
14. Crawford T, Huesgen E, Danziger L. Fidaxomicin: a novel macro cyclic antibiotic for the treatment of Clostridium difficile infection. Am J Health Syst Pharm. 2012;69(11):933-943.
15. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
16. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12(4):281-289.
17. Mikamo H, Tateda K, Yanagihara K, et al. Efficacy and safety of fidaxomicin for the treatment of Clostridioides (Clostridium) difficile infection in a randomized, double-blind, comparative Phase III study in Japan. J Infect Chemother. 2018;24(9):744-752.
18. Guery B, Menichetti F, Anttila VJ, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis. 2018;18(3):296-307.
19. Burton HE, Mitchell SA, Watt M. A Systematic Literature Review of Economic Evaluations of Antibiotic Treatments for Clostridium difficile Infection. Pharmacoeconomics. 2017;35(11):1123-1140.
20. Reveles KR, Backo JL, Corvino FA, et al. Fidaxomicin versus vancomycin as a first-line treatment for Clostridium difficile-associated diarrhea in specific patient populations: a pharmacoeconomic evaluation. Pharmacotherapy. 2017;37(12):1489-1497.
21. Cornely OA, Watt M, McCrea C, Goldenberg SD, De Nigris E. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients aged ≥60 years (EXTEND): analysis of cost-effectiveness. J Antimicrob Chemother. 2018;73(9):2529-2539.
22. Rajasingham R, Enns EA, Khoruts A, Vaughn BP. Cost-effectiveness of Treatment Regimens for Clostridioides difficile Infection: an evaluation of the 2018 Infectious Diseases Society of America Guidelines. Clin Infect Dis. 2020;70(5):754-762.
23. Zinplava (bezlotoxumab) package insert. Whitehouse Station, NJ. Merck & Co. Inc; 2016.
24. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile Infection. N Engl J Med. 2017;376(4):305-317.
25. Gerding DN, Kelly CP, Rahav G, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile Infection in patients at increased risk for recurrence. Clin Infect Dis. 2018;67(5):649-656.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





