US Pharm. 2023;48(5):24-31.
ABSTRACT: In the United States, major depressive disorder (MDD) ranks second among all diseases and injuries as a cause of disability. The American Psychiatric Association clinical practice guideline provides recommendations for the treatment of MDD, with therapy options including selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, atypical antidepressants, monoamine oxidase inhibitors, and others. There is currently no recommendation for the use of one agent over another, as side effects, tolerability, and previous medication trials are typically the driving factors in determining treatment. The recent FDA approval of Auvelity (dextromethorphan-bupropion) provides a fast-acting treatment option for MDD with minimal side effects.
In 2008, major depressive disorder (MDD) ranked as the third cause of disease burden worldwide by the World Health Organization (WHO), and in the United States, it ranks second among all diseases and injuries as a cause of disability.1,2 Moreover, the WHO projects that by 2030, MDD will rank as the number one cause of disease burden across the globe. It is a highly prevalent psychiatric disorder that affects women at a rate almost twice as high as in men. MDD is more common in people without interpersonal relationships and in those who are divorced, separated, or widowed. Individuals who suffer from MDD often have comorbid conditions, such as substance-use disorder, panic disorder, social anxiety disorder, and obsessive-compulsive disorder.2 MDD is diagnosed when an individual has a persistently low or depressed mood, anhedonia (a decreased interest in once-pleasurable activities), feelings of guilt or worthlessness, lack of energy, change in appetite, poor concentration, psychomotor retardation or agitation, sleep disturbances, or suicidal thoughts.2 According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, an individual must be experiencing a depressed mood or anhedonia causing social or occupational impairment, plus four of the other mentioned symptoms, to be diagnosed with MDD.2 MDD is believed to be multifactorial, including biological, genetic, environmental, and psychosocial factors.2
Historically, MDD was considered mainly due to abnormalities in neurotransmitters, especially serotonin, norepinephrine, and dopamine. This has been proven by the use of different antidepressants for treatment, such as selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and dopamine-norepinephrine reuptake inhibitors.2 However, recent theories postulate that MDD is associated primarily with complex neuroregulatory systems and neural circuits, causing secondary disturbances of neurotransmitter systems. Patients suffering from MDD have been found to have lower plasma, cerebrospinal fluid, and brain gamma-aminobutyric acid (GABA) levels. GABA, an inhibitory neurotransmitter, is considered to exert its antidepressant effect by inhibiting the ascending monoamine pathways, including the mesocortical and mesolimbic systems. Drugs that antagonize N-methyl-D-aspartate (NMDA) receptors have also been found to have antidepressant properties.2
In the first year of the COVID-19 pandemic, the prevalence of anxiety and depression increased globally by 25%, according to the WHO.3 Today, many people remain unable to get the care and support that they need for both preexisting and newly developed mental health conditions. Stress is a well-established contributor to the development, onset, and severity of mental health disorders.4 Multiple stress factors may have contributed to increased anxiety and depression, including social isolation, constraints on people’s ability to work, fear of infection, grief, exhaustion, and financial worries during the pandemic.3
MDD can be managed with various treatment modalities, including pharmacologic, psychotherapeutic, interventional, and lifestyle modifications. Initial treatment of MDD includes medications and/or psychotherapy. FDA-approved medications for the treatment of MDD include SSRIs, SNRIs, serotonin modulators, atypical antidepressants, tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), and other mood stabilizers.2 All antidepressants are believed to be equally effective but differ in their side-effect profiles. Treatment is usually based on the patient’s response and tolerability of medications. Three commonly used methods of psychotherapy include cognitive behavior therapy, interpersonal therapy, and supportive therapy. This article will focus on Auvelity (dextromethorphan-bupropion), a recently approved fast-acting medication for the treatment of MDD.
Depression Rating Scales
Rating scales are tools commonly used in psychiatric conditions to evaluate symptoms and measure the effectiveness of treatments. The British Journal of Psychiatry recommends the use of two rating scales in the management of depression: the Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Asberg Depression Rating Scale (MADRS). The HAM-D is the most widely used numerical scale designed to screen for depression. The 17 items assessed include depressed mood, feelings of guilt, suicide, insomnia (early, middle, and late), work and activities, retardation, agitation, anxiety (psychological and/or somatic), gastrointestinal somatic symptoms (i.e., appetite), general somatic symptoms, sexual dysfunction, hypochondrias, weight loss, and insight. The classification of symptoms that may be difficult to obtain is scored on a scale of 0 to 2 (0-absent, 1-doubtful, 2-present). Classification of symptoms where more detail can be obtained is expanded on a scoring system of 0 to 4 (0-absent, 1-mild, 2-moderate, 3-severe, 4-incapacitating). Generally, the higher the score, the more severe the depression. Interpreting the HAM-D total score level of depression is defined as 0 to 13 mild, 14 to 17 mild to moderate, and >17 moderate to severe.12
The MADRS rating is based on a clinical interview, wherein the rater must decide if the score lies on the defined scale—either from 0 to 6 or 1 to 5, depending on the symptoms being evaluated. The 10-question assessment consists of screening for apparent sadness, reported sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, inability to feel, pessimistic thoughts, and suicidal thoughts. Interpreting the results of the MADRS rating scale is categorized by: 0 to 8, meaning depressive symptoms are absent; 9 to 17, mild depression; 18 to 34, moderate depression; and 35 to 60, severe depression.13 Additional rating scales are available for measuring symptom severity in MDD, including patient health questionnaire (PHQ-2 and PHQ-9), Quick Inventory of Depression Scale–Self Report (QIDS-SR), Children’s Depression Rating Scale, Edinburgh Postnatal Depression Scale, and Geriatric Depression Rating Scale.
Auvelity
Auvelity is the first fast-acting medication FDA-approved for the treatment of MDD.5 This combination therapy is manufactured by Axsome Therapeutics and formulated as an extended-release tablet comprised of 45 mg of dextromethorphan and 105 mg of bupropion. Dextromethorphan is a noncompetitive, nonselective NMDA receptor antagonist (ionotropic glutamate receptor) that mediates excitatory neurotransmission in the brain. Dextromethorphan is also a sigma-1 receptor agonist that is known for its antitussive effects; however, this pathway is thought to additionally modulate monoamine and glutamate signaling in the central nervous system.5 Dextromethorphan is rapidly metabolized via hepatic demethylation by CYP2D6 to its active form, dextrorphan. Although the role of bupropion in Auvelity is unclear, there are two mechanisms that may contribute to its use. Bupropion is a selective norepinephrine and dopamine reuptake inhibitor and a strong inhibitor of CYP2D6. When bupropion is combined with dextromethorphan, a drug-drug interaction is observed. This intentional interaction increases the overall concentration of dextrorphan in the central nervous system, prolonging its effects.
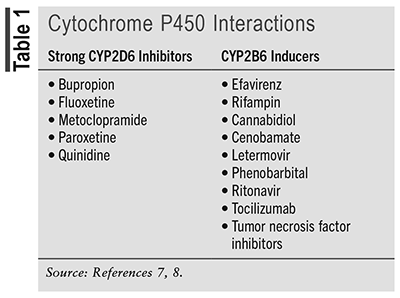
The package insert for dextromethorphan-bupropion (Auvelity) recommends a dosage of one tablet given daily in the morning for 3 days, then increasing to one tablet twice daily given at least 8 hours apart for a total daily dosage of 90 mg of dextromethorphan and 210 mg of bupropion. Dextromethorphan-bupropion may be taken with or without food, and the tablet should be swallowed whole, not crushed, chewed, or divided, due to its extended-release formulation. Dose adjustments should be made in patients with renal impairment, in those with known genetic polymorphisms, and in patients who are taking other medications that utilize the cytochrome P450 system for metabolism.5 Patients with moderate renal impairment, or an estimated glomerular filtration rate (eGFR) of 30 to 49 mL/min, should take one tablet daily. Dextromethorphan-bupropion is not recommended in patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2). It is recommended to prescribe one tablet daily in patients who are CYP2D6 poor metabolizers or who are taking other strong CYP2D6 inhibitors. Its use is not recommended in combination with strong CYP2B6 inducers. Refer to TABLE 1 for examples of medications that should be avoided when taking dextromethorphan-bupropion.
The most common adverse reactions reported with dextromethorphan-bupropion include dizziness, headache, diarrhea, somnolence, dry mouth, and hyperhidrosis.6 Dextromethorphan-bupropion also has a black box warning of suicidal thoughts and behaviors in pediatric and young adult patients and, therefore, was not FDA-approved for use in this population. Other contraindications include seizure disorder, alcohol/substance-use disorder, and bipolar disorder due to bupropion having an increased risk of causing seizures and current or prior diagnosis of bulimia.6 The package insert also discourages the use of MAOIs in combination or within 14 days of taking Auvelity.
Dextromethorphan-bupropion was FDA-approved in response to the positive results of two trials. The GEMINI (Glutamatergic and Monoaminergic Modulation in Depression) trial was a phase III, double-blind, placebo-controlled study in 327 patients with MDD.9 Inclusion criteria included age 18 to 65 years and a MADRS total score of 25 or higher. Exclusion criteria included bipolar disorder, psychotic disorder, panic disorder, obsessive-compulsive disorder, treatment-resistant depression, alcohol/substance-use disorder within the past year, significant risk of suicide, and a history of seizure disorder. The participants were randomly assigned (1:1 ratio) to receive dextromethorphan-bupropion or placebo for 6 weeks. The primary endpoint was symptom improvement on the MADRS total score at 6 weeks, and secondary endpoints were changes in MADRS score from baseline to Weeks 1 and 2, clinical response at Week 6, and remission at Week 2. The results of the trial showed that dextromethorphan-bupropion was superior to placebo in terms of MADRS improvement at all time points. Remission was achieved by 39.5% of patients treated with dextromethorphan-bupropion versus 17.3% of patients with placebo at Week 6. Reported adverse events included dizziness, nausea, headache, somnolence, and xerostomia. Of note, the use of dextromethor-
phan-bupropion was not associated with weight gain or increased sexual dysfunction as commonly seen with other agents used in the treatment of MDD.9
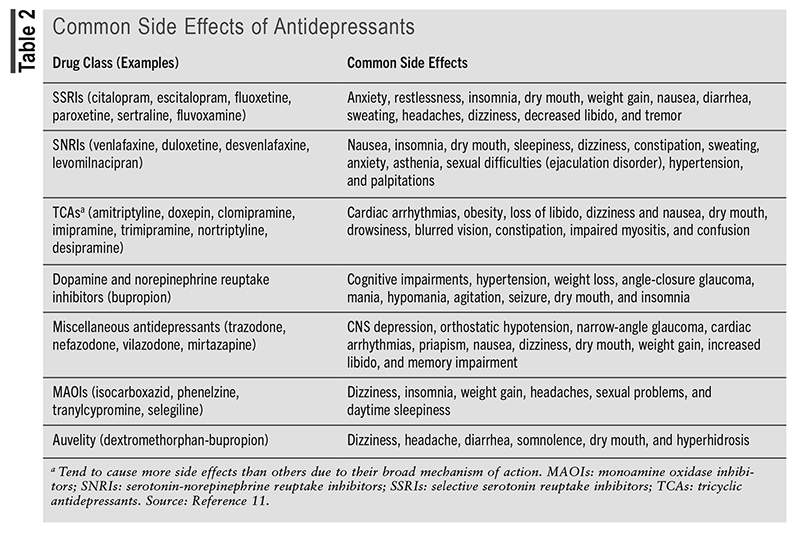
The ASCEND (Assessing Symptomatic Clinical Episodes in Depression) trial was a phase II, randomized, double-blind, multicenter, parallel-group study.10 Ninety-seven participants were randomly assigned (1:1 ratio) to receive either 45-mg dextromethorphan and 105-mg bupropion twice daily or 105-mg bupropion sustained release twice daily for a total of 6 weeks. Inclusion criteria included patients aged 18 to 65 years diagnosed with moderate or greater severity of MDD. The primary endpoint measured was the change in MADRS score from baseline to Week 6. The mean change in MADRS score in the dextromethorphan-bupropion group was significantly improved compared with the bupropion sustained-release group (-13.6 vs. -8.8 points). Additionally, a greater number of patients treated with dextromethorphan-bupropion reached remission at Week 2 and all other points afterward. Common adverse events reported included dizziness, xerostomia, nausea, anxiety, and decreased appetite. Refer to TABLE 2 for common side effects of other commonly prescribed antidepressants.
Although it is unclear, dextromethorphan is thought to be habit-forming. There is an alarming number of abuse reports of dextromethorphan being taken for psychoactive effects. People who overdose on dextromethorphan can experience symptoms similar to those caused by PCP or ketamine.15 Intoxication can present with behavioral and physical symptoms, including somnolence, hallucinations, tachycardia, seizures, and slow breathing. The misuse of dextromethorphan has resulted in many states now requiring a form of identification to prove purchasers are aged at least 18 years. According to the National Alliance on Mental Illness, there are no risks associated with long-term use of bupropion.16
Role of the Pharmacist
Pharmacists are one of the most accessible healthcare workers available to make treatment recommendations and offer their expertise in the safe use of OTC and prescription medications. Pharmacists play a vital role in counseling patients on side effects and drug interactions. They also have the knowledge necessary to interpret and determine the clinical severity of a drug interaction. As dextromethorphan-bupropion begins to make its way to the shelves of pharmacies, pharmacists should be prepared to educate patients who are prescribed this medication on the side effects and components of this combination therapy. Patients should be counseled to avoid OTC cough suppressants that contain dextromethorphan as too much dextromethorphan can cause intoxication. A person is likely to become intoxicated at a dose of 1,500 mg or more. Symptoms of this include impaired judgment, hallucinations, agitation, nausea or vomiting, tachycardia, changes in blood pressure, and slow breathing. Intoxication can occur between 15 and 30 minutes after ingestion, and effects can last from 3 to 6 hours.15 If an individual suspects dextromethorphan intoxication, emergency personnel should be contacted as soon as possible. In addition, with the increased cost that comes with many new-to-market medications, patients and prescribers may ask if taking bupropion SR and OTC dextromethorphan will have the same rapid response in the treatment of MDD. The answer to that question is unknown, as it has not been studied. OTC dextromethorphan products often come in combination with other agents that would not be necessary for treating MDD. Also, these products are not always extended release, which is how it is formulated in the combination dextromethorphan-bupropion. To match the dosing of dextromethorphan-bupropion, the patient would have to dose OTC dextromethorphan three times daily, which exceeds the recommended dose on the package. Lastly, many medications utilize the CYP2D6 enzyme for metabolism. Pharmacists should feel confident in their ability to catch these drug-drug interactions and counsel the patient on the risks.
Despite the advantages of dextromethorphan-bupropion in clinical trials, there is still concern about the pricing of this new medication. The drug unit cost is $20.96, which would make a 1-month supply (60 tablets) more than $1,200.14 Insurance companies may be hesitant to add dextromethorphan-bupropion to their formularies until guidelines are updated because less expensive agents are still currently guideline-recommended by psychiatrists for the treatment of MDD.
Conclusion
Studies have shown that mental illness is on the rise worldwide. Scientists are working hard to research and develop new medications that will provide symptom relief faster than the currently approved medications for MDD. Dextromethorphan-bupropion is a potential alternative for patients who are seeking fast-acting symptom improvement or are unable to tolerate the side effects of other antidepressants. In addition, the general public needs to make an ongoing effort towards reducing the stigma that exists around mental illness. Healthcare providers play a major role in providing resources and treatment options for patients suffering from various mental health conditions. Pharmacists are well versed in the medications, side effects, and drug interactions available for MDD, making them a good resource for provider and patient education. They can also partake in the role of helping patients with mental health illnesses by being aware of local crisis centers and providing their patients with realistic goals of therapy.
REFERENCES
1. Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591-608.
2. Bains N, Abdijadid S. Major depressive disorder. Updated June 1, 2022. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. www.ncbi.nlm.nih.gov/books/NBK559078/.
3. World Health Organization. COVID-19 pandemic triggers 25% increase in prevalence of anxiety and depression worldwide. March 2, 2022. www.who. int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide. Accessed April 21, 2023.
4. Haider II, Tiwana F, Tahir SM. Impact of the COVID-19 pandemic on adult mental health. Pak J Med Sci. 2020;36(COVID19-S4):S90-S94.
5. Auvelity (dextromethorphan HBr and bupropion HCl) prescribing information. New York, NY: Axsome Therapeutics, Inc.
6. Bupropion/dextromethorphan (contained in: Auvelity). www.micromedexsolutions.com.
7. Straight Healthcare. Cytochrome P450 2D6. www.straighthealth¬care.com/cytochrome-p450-2d6.html. Accessed February 19, 2023.
8. Straight Healthcare. Cytochrome P450 2B6. www.straighthealth¬care.com/cytochrome-p450-2b6.html. Accessed February 19, 2023.
9. Iosifescu DV, Jones A, O’Gorman C, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83(4):21m14345.10. Tabuteau H, Jones A, Anderson A, et al. Effect of AXS-05 (dextro¬methorphan-bupropion) in major depressive disorder: a randomized double-blind controlled trial. Am J Psychiatry. 2022;179(7):490-499.
11. Ramic E, Prasko S, Gavran L, Spahic E. Assessment of the antidepressant side effects occurrence in patients treated in primary care. Mater Sociomed. 2020;32(2):131-134.
12. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278-296.
13. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
14. Dextromethorphan/bupropion (Auvelity). In: Lexi-Drugs. Hudson, OH: Lexicomp, Inc; 2022. http://online.lexi.com. Accessed February 18, 2023.
15. American Addiction Centers. Dextromethorphan/DXM overdose: dangers of abusing cough med-icine. 2023. https://americanaddictioncenters.org/dextromethorphan-dxm. Accessed April 21, 2023.
16. NAMI. Bupropion (Wellbutrin). www.nami.org/About-Mental-Illness/Treatments/Mental-Health-Medications/Types-of-Medication/Bupropion-(Wellbutrin). Accessed April 21, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






