US Pharm. 2020;45(10):6-8.
Triple-negative breast cancer (TNBC) is a term that originally applied to cancers that lack expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). This means that TNBC is not stimulated from exposure to the female hormones estrogen or progesterone, nor through an overactive HER2 pathway.1
TNBC tends to behave more aggressively than other types of breast cancer. Many available and effective treatment options for the majority of breast cancers block the growth-stimulating effects of ER, PR, or HER2; therefore, TNBC has had limited therapeutic options. TNBC is often diagnosed at a more advanced stage and proportionately affects younger women more often than other breast cancers. Novel treatment options for TNBC have lagged behind those of other types of breast cancers. Currently, immunotherapy in combination with chemotherapy is available for those with advanced TNBC that expresses programmed cell death ligand 1 (PD-L1).1,2
Risk factors associated with the diagnosis of TNBC include positive BRCA mutation status, premenopausal status, obesity, and a young age at first pregnancy. TNBC accounts for approximately 12% to 15% of breast cancers diagnosed worldwide, which amounts to almost 200,000 cases each year.2
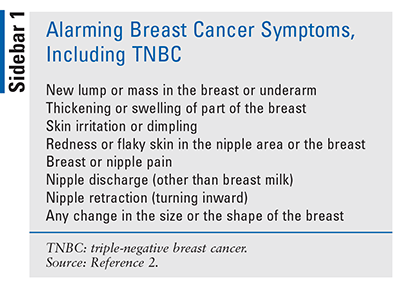
All of the symptoms described in SIDEBAR 1 occur because cancer cells grow uncontrollably in different parts of the breast (BRCA1). The three main parts of the breast are the lobules, ducts, and connective tissue. The lobules are the glands that produce milk. The ducts are tubes that carry milk to the nipple. The connective tissue, which consists of fibrous and fatty tissue, surrounds and holds all of these parts together. Most breast cancers begin in the ducts or lobules and can spread outside the breast through blood vessels and lymph vessels.2
NEW DEVELOPMENTS IN TREATING TNBC
The aim of treatment is to palliate symptoms, prolong survival, and maintain quality of life. The development of more effective treatment for TNBC requires new and innovative therapies to be evaluated in TNBC patients.
Checkpoint Inhibitors
Programmed cell death 1 (PD-1) is a protein that inhibits certain types of immune responses. Drugs that block PD-1 protein are called checkpoint inhibitors and may enhance the ability of the immune system to fight cancer. This kind of immunotherapy has generated a lot of hope and excitement for its ability to help the immune system recognize and attack cancer.
Keytruda (pembrolizumab): Pembrolizumab is a fully humanized monoclonal antibody checkpoint inhibitor that binds with high affinity to the PD-1 receptor. In heavily pretreated patients with recurrent or metastatic TNBC positive for PD-1, Keytruda has been reported to produce a response rate of 18.5%. A trial directly comparing Keytruda with chemotherapy in recurrent TNBC found that Keytruda was not better, but results in advanced breast cancer have been mixed.3
Keytruda in combination with standard therapy as neoadjuvant treatment for patients with locally advanced TNBC increased the pathologic complete response (pCR) nearly threefold. Neoadjuvant chemotherapy is a type of chemotherapy done before surgery with the goal of reducing the size of the tumor so that it is less invasive, resulting in more effective surgical outcomes. Adjuvant chemotherapy is administered after surgery to kill any remaining cancer cells with the goal of reducing the chances of recurrence. Neoadjuvant Keytruda has been reported to be significantly more effective than chemotherapy at eradicating cancer confined to the breast prior to surgery.4
Overall, analyses have revealed that Keytruda-treated patients were less likely to have evidence of cancer in their surgically removed breast tissue and more likely to survive without evidence of cancer recurrence. PD-L1–positive patients had a higher response to chemotherapy and combination treatment with Keytruda without unexpected side effects. PD-L1 is a 40kDa type 1 transmembrane protein.4
Initial results from the KEYNOTE-355 clinical trial in previously untreated advanced TNBC were released at the 2020 American Society of Clinical Oncology Annual Meeting. Overall, 847 patients were treated with Keytruda plus chemotherapy or chemotherapy alone and directly compared. Patients were evaluated based on their combined positive score (CPS), which measures the amount of PD-L1 expression on cancer cells.3,8 In patients with the highest CPS of 10 or greater, the addition of Keytruda to chemotherapy significantly prolonged survival without cancer progression to 9.7 months, compared with 5.6 months for chemotherapy alone. At 1 year, 39.1% of patients treated with Keytruda survived progression-free, compared with 23% for those treated with chemotherapy. Among patients with CPS of 10 or greater, the median progression-free survival was 7.6 months for Keytruda plus chemotherapy, compared with 5.6 months for chemotherapy alone.3
Tecentriq (atezolizumab): Atezolizumab is another checkpoint inhibitor, and when combined with paclitaxel in women with advanced TNBC, it produces an anticancer response in 70.8% of patients. The combination of Tecentriq and paclitaxel improved average survival duration among patients with PD-L1–positive tumors to 25 months, compared with 15.5 months for paclitaxel alone, leading to accelerated FDA approval.5,6
Treatment combinations consisting of checkpoint inhibitors plus paclitaxel and other known active drugs in TNBC, such as gemcitabine and carboplatin, are ongoing to determine the optimal method to incorporate this new class of drugs into the overall management of TNBC.7
Antibody-Drug Conjugates
Antibody-drug conjugates (ACDs) represent a new class of highly potent anticancer drugs. An antibody that is highly selective for a tumor-associated antigen is used. The cytotoxic agent cannot be administered alone due to toxicity. However, when the cytotoxic agent is attached in small amounts to the antibody by a linker that is stable in circulation, the linker releases the cytotoxic agent into the target cells by lysosomal degradation.
Kadcyla (ado-trastuzumab emtansine): One new ACD that has been FDA- and EMA-approved for breast cancer is Kadcyla for HER2-positive disease, and more of these medications are in development.8
Trodelvy (sacituzumab govitecan-hziy): Trodelvy is an ACD and an immune-targeted therapy medication. It is comprised of an antibody that attaches to specific receptors called Trop-2 receptors. The antibody is attached to a drug that kills cancer cells, called SN-38.8
Trop-2 receptors are often found in large numbers on the surface of cancer cells, but not on healthy cells. Once Trodelvy binds to the Trop-2 receptors, it delivers SN-38 into the cancer cell. This kills the cancer cell, while only affecting a small portion of healthy cells. By targeting Trop-2 receptors, larger amounts of chemotherapy can be delivered to the cancer cells because healthy cells are largely spared from the cancer-killing effects of the treatment. Trodelvy is now approved by the FDA for TNBC.8
PARP Inhibitors
Poly (ADP-ribose) polymerase (PARP) is a protein that has a role in cellular growth, regulation, and cell repair, which helps the cancer cells repair themselves and survive. PARP inhibitors are a family of targeted therapeutic agents that have recently been approved by the FDA for 10% to 15% of TNBC patients with germline mutations in BRCA1/2. PARP inhibitors target the DNA repair process and have two main mechanisms of action: synthetic lethality and PARP-DNA trapping.9
The efficacy of two PARP inhibitors, olaparib and talazoparib, was recently demonstrated in two independent phase III randomized, controlled trials in metastatic HER2-negative BRCA1/2-mutated patients who received fewer than three lines of chemotherapy. These trials demonstrated comparable results in which the PARP inhibitor was associated with an improvement in progression-free survival of about 3 months and an objective response rate near 60% compared with single-agent chemotherapy.1,10
MEK Inhibitors
Recent studies looking at mitogen-activated protein kinase (MEK) pathway inhibitors in TNBC have generated some encouraging activities. The combination of MEK inhibitor (cobimetinib) with other therapies such as atezolizumab (checkpoint inhibitor) and either paclitaxel or nab-paclitaxel in PD-L1–positive patients had response rates of 44% with paclitaxel combination versus 33% with nab-paclitaxel. Researchers are now focusing on the results of more trials with MEK inhibitors in combination with other therapies.11
NEW COMBINATION STRATEGIES
Adding neoadjuvant carboplatin to presurgery chemotherapy improved disease-free survival for patients with TNBC. Previously reported clinical study results have suggested that adding carboplatin to anthracycline/taxane-based neoadjuvant chemotherapy can increase the proportion of patients with TNBC who had attained a pCR, from 36.9% to 53.2%.12
In a recently reported study, researchers enrolled 315 patients with TNBC to receive 18 weeks of neoadjuvant chemotherapy consisting of paclitaxel, nonpegylated-liposomal doxorubicin, and bevacizumab who were randomly assigned to concurrently receive weekly carboplatin or nothing additional.11 After a median follow-up of 3 years, 85.5% of TNBC patients treated with the additional carboplatin survived without evidence of cancer recurrence, compared with only 76.1% of patients treated with paclitaxel, nonpegylated-liposomal doxorubicin, bevacizumab, and no carboplatin.12
Atezolizumab/nab-paclitaxel is currently FDA-approved for PD-L1–positive TNBC.13
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Nanda R, Chow LQ, Dees EC, et al. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. Presented at the 2014 San Antonio Breast Cancer Symposium, December 9-13, 2014. San Antonio, Texas.
2. Triple Negative Breast Cancer (TNBC). www.webmd.com/breast-cancer/triple-negative-breast-cancer#1.
3. Merck’s KEYTRUDA (pembrolizumab) in combination with chemotherapy met primary endpoint of pathological complete response (pCR) in pivotal phase 3 KEYNOTE-522 trial in patients with triple-negative breast cancer (TNBC).
4. Schmid P, Cortés J, Dent R, et al. KEYNOTE-522: Phase III study of pembrolizumab + chemotherapy vs placebo + chemo as neoadjuvant treatment, followed by pembrolizumab vs placebo as adjuvant treatment for early triple-negative breast cancer. ESMO Congress 2019. Presented September 29, 2019.
5. Adams S, Diamond J, Hamilton E, et al. Safety and clinical activity of atezolizumab (anti-PD-L1) in combination with nab-paclitaxel in patients with triple-negative breast cancer. Proceedings from the 2015 annual San Antonio Breast Cancer Symposium. Presented December 10, 2015.
6. Adams S, Diamond JR, Hamilton EP et al. Phase Ib trial of atezolizumab (anti-PD-L1) in combination with nab-paclitaxel in patients with triple-negative breast cancer. J Clin Oncol. 2016:34(suppl).
7. Cortes J, Cescon DW, Rugo HS, et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. Presented at ASCO20 Virtual Scientific Program. J Clin Oncol. 2020;38 (suppl).
8. Immunomedics, Inc. Press Release. FDA Grants Breakthrough Therapy Designation to Immunomedics for Sacituzumab Govitecan for the Treatment of Patients with Triple-Negative Breast Cancer. www.immunomedics.com/news-2016.shtml.
9. Sonnenblick A, Azambuja ED, Hatem A, et al. An update on PARP inhibitors-moving to the adjuvant setting. Nat Rev Clin Oncol. 2015;12:27-41.
10. McCann KE, Hurvitz SA, Advances in the use of PARP inhibitor therapy for breast cancer. Drugs Context. 2018;7:212540.
11. Duncan JS, Whittle MC, Nakamura K, et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149(2):307-332.
12. Castrellon AB, Pidhorecky I, Valero V, The role of carboplatin in the neoadjuvant chemotherapy treatment of triple negative breast cancer. Oncol Rev. 2017;11(1):324.
13. Promising New Treatments for Triple-Negative Breast Cancer: Immunotherapy and Other Targeted Therapies. The ASCO Post. www.ascopost.com/issues/december-10-2019/potential-new-treatments-for-triple-negative-breast-cancer/
To comment on this article, contact rdavidson@uspharmacist.com.






