Peptic ulcer disease (PUD) is defined as an erosion in the lining of the stomach or the duodenum. "Peptic" alludes to pepsin, a proteolytic enzyme that catalyzes the hydrolysis of proteins.1,2 About 4% to 10% of Americans develop PUD at some point. PUD incidence increases with age, with PUD most common in those older than 40.3,4
Large ulcers are usually noticeable because they are more likely to cause serious bleeding, while smaller ulcers may not cause symptoms. Most ulcers occur within the first layer of the gastrointestinal (GI) tract from a perforation. In some cases, this perforation may completely penetrate the intestinal lining, constituting a medical emergency. Although PUD has a variety of causes, including stress and NSAID use, it is most frequently associated with Helicobacter pylori . This bacterium accounts for about 90% of diagnosed cases.
Etiology and Pathophysiology
Predisposing factors for PUD include chronic NSAID use, cigarette smoking, hypersecretion of gastric acid, viral infections, vascular insufficiency, radiation treatment, corticosteroid use, a diet that is spicy or high in caffeine, chemo therapy, alcohol use, and rare genetic subtypes.1-3
Although there are several causative mechanisms for PUD, this column focuses on H. pylori. Several theories have been proposed to explain how H. pylori produces mucosal injury. One involves urease. Specifically, urease catalyzes urea into ammonia, enabling H. pylori to survive in the acidic environment of the stomach. Additionally, urease may erode the mucosal barrier, leading to epithelial cell damage. Mucolytic enzymes (e.g., bacterial protease, lipase) appear to be involved in the degradation of the mucous layer and make the epithelium more susceptible to acid damage. Produced by H. pylori and implicated in host epithelial cell damage, cytokines and other inflammatory mediators may be noted in response to inflammation and have a role in mucosal damage and subsequent ulcerogenesis.1-5
Signs and Symptoms
Patients with PUD may notice nausea, bloating, vomiting (sometimes vomit contains blood), burping, loss of appetite, anorexia, and/or epigastric pain that radiates to the back.1-3 Other signs and symptoms include heartburn, indigestion, and abdominal fullness.
Diagnostics
Methods of diagnosing H. pylori infection include upper GI tract biopsy, histological examination, rapid urease test, culture, antibody detection, antigen detection, and the urea breath test. When endoscopy is indicated, a histological examination is the diagnostic test of choice.1 Due to a lack of sensitivity, culture is indicated only for antibiotic-resistance testing in patients who are unresponsive to therapy. Laboratory serology methods are more sensitive than those available in the physician's office; however, since they cannot distinguish between an active and a resolved infection, they are not recommended for monitoring or confirming the eradication of the organism. The stool antigen and urea breath tests have similar accuracy and are preferred when endoscopy is not clinically indicated.4,6,7
BreathTek
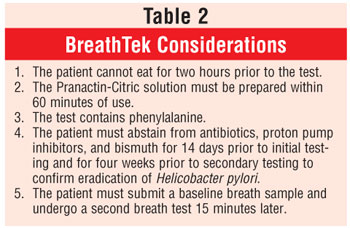
Developed by Meretek Diagnostics, Inc., BreathTek is an FDA-approved diagnostic device that uses breath samples to detect H. pylori. The diagnostic drug component of BreathTek is 13C-urea, a synthetic urea contained in a granulated powder that is mixed with water and consumed by the patient. In the presence of urease, 13C-urea decomposes to carbon dioxide (13 CO2) and ammonium. The 13CO2 is absorbed in the blood and exhaled in the breath, resulting in an increase in the ratio of 13CO2 to 12CO2.6 Consequently, when compared to baseline, if there is an increase in the ratio of 13CO2 to 12CO2 in a postdose breath sample, the results are deemed positive for H. pylori infection. The analyses of the breath samples are performed by a spectrophotometer, the UBiT-IR300 or POCone, and results are confirmed in about two minutes. Patient instructions and device considerations are found in tables 1 and 2, respectively.
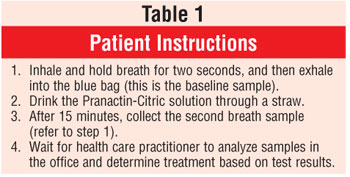
BreathTek is indicated for patients with active PUD, a history of documented PUD, and/or mucosal-associated lymphoid-type lymphoma. Testing is not recommended for asymptomatic individuals lacking PUD history or for those on long-term proton pump inhibitor therapy. Moreover, testing should be performed only when H. pylori is suspected.4,6,7
Clinical Efficacy
Data collected from a clinical trial conducted in Europe and the United States indicated that BreathTek was excellent in the detection of H. pylori. Patients referred for an upper GI endoscopy were eligible to enter the study, regardless of whether they had been previously diagnosed with an ulcer. Of the 432 patients enrolled, 393 successfully completed the study. Results for H. pylori infection were positive in 225 patients, negative in 156 patients, and indeterminate in 12. Participants were also tested using histopathologic methods; results showed that BreathTek was superior in detecting H. pylori .6
Another prospective study was performed to determine the accuracy of various tests used in the diagnosis of H. pylori infection. This study included 314 patients who were referred to an endoscopy center for an upper endoscopy. The diagnostic exams included the rapid urease test (RUT), histology, culture, 13CO2 breath test (UBT), and serum immunoglobulin G (EIA). Patients were deemed to have positive results if the culture or at least two of the other tests were positive. The prevalence of H. pylori infection in this population was 72%. The diagnostic test that had greatest efficacy was the UBT (sensitivity, 97%; specificity, 100%). EIA displayed good sensitivity (96%), but it was the test with the least specificity (71%). RUT, histology, and culture demonstrated high specificity (>98%); however, their sensitivity was lower than 90%. In elderly patients (age >65 years, n = 120), UBT had the greatest combination of sensitivity (94%) and specificity (100%).8
Conclusion
Indicated for the initial diagnosis and posttreatment monitoring of H. pylori infection, Breath-Tek has demonstrated 95% sensitivity in detecting active infection, costs $103 per test, and is a reimbursable procedure.5,6 For more information, contact Meretek at www.meretek.com or (888) 637-3835.
References
1. Beers M, Berkow R. The Merck Manual. 17th ed. West Point, PA: Merck Research Laboratories; 1999.
2. Green G. The Washington Manual of Medical Therapeutics. 31st ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2004.
3. DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. New York, NY: McGraw Hill; 2002.
4. Medline Plus. Peptic Ulcer. Available at: www.nlm.nih.gov/medlineplus/ency/ article/000206.htm. Accessed September 8, 2005.
5. Peptic Ulcer Disease (PUD). Diseasedex--General Medicine Clinical Reviews: Micromedex, Inc.; September 31, 2005.
6. BreathTek. BreathTek: Physician Information. Available at: www.meretek.com/dr_info.html. Accessed on September 8, 2005.
7. Quest Diagnostics. Helicobacter pylori Urea Breath Test (UBiT). Available at: www.questdiagnostics.com/hcp/intguide/jsp/showintguidepage.jsp?fn=TS_Hpylori_UBiT.htm. Accessed on September 8, 2005.
8. Gomollon F, Ducons JA, Santolaria S, et al. Breath test is very reliable for diagnosis of Helicobacter pylori infection in real clinical practice. Dig Liver Dis. 2003;35:612-618.
To comment on this article, contact
editor@uspharmacist.com.






