US Pharm. 2009;34(5)(Oncology suppl):6-12.
ABSTRACT: The term breast carcinoma describes a diverse group of cancerous lesions that differ in microscopic appearance and biological behavior. For this reason, knowledge about the pathology of the patient-specific breast carcinoma is vital in order to determine the characteristics of the tissue sample and to properly diagnose and treat the patient.1-3 This article provides a brief review of the causes, risk factors, clinical presentation, screening, and diagnosis of breast cancer; explains the various components of the pathology examination and their impact on disease pathogenesis; and discusses how gene expression profiling can provide further prognostic and predictive information on the clinical outcomes of breast cancer.
Breast cancer is the most common cancer among American women.4-6 It ranks as the second-leading cause of cancer death in women after lung cancer, and it is the leading cause of cancer death among women ages 45 to 55.6-8 Starting in the early 1980s, breast cancer rates rose annually by approximately 3% per year, most likely due to increased availability and use of mammography screening.4,5 Since 1987, incidence rates have leveled, and there was actually a decrease in incidence of 3.5% per year from 2001 to 2004.6,7,9 Today a woman’s lifetime probability of developing breast cancer is 1 in 6 (16.7%) overall, and 1 in 8 (13.4%) for invasive breast cancer.4,6,7,9 The National Cancer Institute estimated that in 2008, more than 182,000 women will be diagnosed with breast cancer, and approximately 40,480 women will die from the disease.7,10
Breast Cancer Basics
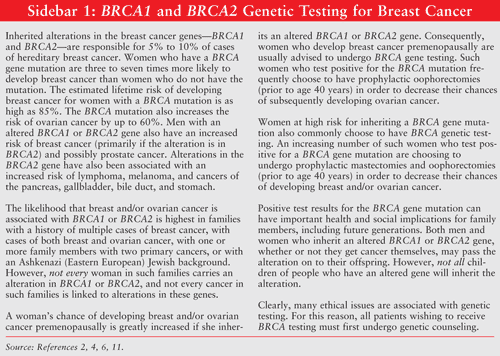
Causes and Risk Factors: While the exact cause of breast cancer is unknown, data suggest that several factors may contribute to the development of the disease.10 Both age and gender are among the strongest risk factors for breast cancer.6,9 Breast cancer occurs 100 times more often in women than in men.9 Incidence rates rise sharply with age until about the age of 45 to 50 years. Caucasian women have the highest incidence of breast cancer, followed by African Americans, Asian Americans/Pacific Islanders, Hispanic/Latinos, and American Indians/Alaskans.7 Women who have a personal or family history of breast cancer are, respectively, 5% and 10% more likely to develop breast cancer.4,6,7,9,11 Genetic testing is available for the breast cancer genes (BRCA1 and BRCA2)—the most common genetic mutation that predisposes patients to breast cancer.8(SIDEBAR 1 discusses BRCA genetic testing for breast cancer.) Endocrine factors (including early age of menarche, late age of menopause, irregular menstrual patterns, and/or infertility) also appear to increase risk.6 Not surprisingly, prolonged exposure to and higher concentrations of endogenous estrogens also increase the risk of breast cancer.4,9Other factors that may increase the risk of developing breast cancer include exposure to industrial chemicals, consumption of high-fat diets, lower socioeconomic status, and obesity.6,7
Clinical Presentation: A painless lump is the initial sign of breast cancer in most women. The typical malignant mass is solitary, unilateral, solid, hard, irregular, and nonmobile. In a small number of cases, stabbing or aching pain is the first symptom. Less commonly, nipple discharge, retraction, or dimpling may herald the onset of the disease. In more advanced cases, prominent skin edema, redness, warmth, and induration of the underlying tissue may be observed.6 Breast cancers that are found because they can be felt tend to be larger and are more likely to have already spread beyond the breast. In contrast, breast cancers found during screening exams are more likely to be small and still confined to the breast.12
Screening: Only 30% of women have one or more identifiable risk factors for breast cancer.4 This underscores the importance of breast cancer screening initiatives. The American Cancer Society’s recommendations regarding breast cancer screenings are discussed in SIDEBAR 2.12

Clinical breast examinations are an important first step in screening for breast cancer. During this examination, special attention is given to the shape and texture of the breasts, the presence of any lumps, and whether such lumps are attached to the skin or to deeper tissues. The areas under both arms are also examined. During the clinical breast examination, women should be taught by their health care professionals the proper technique for doing their own monthly breast examinations.12
Mammograms, which are x-rays of the breast, are considered the best screening tools for detecting breast cancer, although they detect only 85% to 90% of biopsy-proven cancers.4 False-negatives discovered on mammograms are more common in younger women (usually because their breasts are dense, which can hide tumors), in pregnant women, in women who are breast-feeding, in and women who have breast implants.4,6,12 It is important to note that mammography is not a substitute for tissue sampling and histologic evaluation of palpable abnormalities, nor is it a substitute for clinical breast examinations.4
Breast ultrasounds are commonly used in conjunction with mammograms.6,12 Generally speaking, breast ultrasounds are used to target specific areas of concern found on mammograms. Breast ultrasounds, which can help to distinguish between cysts and solid masses, are particularly useful in women who have very dense breasts. It is important to note that breast ultrasounds should not be considered a replacement for mammograms, because they can miss suspicious areas detected on mammograms.12
Breast MRIs—using the contrast medium gadolinium—are also commonly used in conjunction with mammograms to screen women who have a high risk of developing breast cancer, or to better examine suspicious areas found by mammograms.6,12 Breast MRIs are also commonly used for women who have been diagnosed with breast cancer to better determine the actual size of the tumor and to look for any other tumors in the breast. Breast MRIs are more sensitive in detecting cancers than mammograms, but are associated with an extremely high false-positive rate. Not surprisingly, breast MRIs are much more expensive than mammograms.12
Diagnosis: Breast biopsies are indicated for mammographic abnormalities that suggest malignancy and for palpable masses on physical exam.6 Breast cancer cannot be diagnosed without a breast biopsy.12 The most common types of breast biopsies are fine-needle aspiration, core-needle biopsy, and excisional biopsy.6,12 Core-needle biopsies are generally the preferred method, because they offer a more definitive histological diagnosis, avoid inadequate samples, and can distinguish between invasive versus noninvasive cancers.6
Information Contained in the Pathology Report
Routine pathologic evaluation remains the most critical element in determining the prognosis of patients with breast cancer.3 The pathologic evaluation of breast tumors serves to establish the histologic diagnosis and to confirm the presence or absence of other factors believed to influence the prognosis.6 Furthermore, information found in the pathology report aids in staging breast cancer. Information commonly found on the pathology report includes anatomical site, tumor size, tumor type, histopathologic grade of tumor, surgical margins, lymph node involvement, lymphovascular invasion, hormone receptor status, the Ki-67 proliferation index, and HER2/neu receptor status. It is important to note that all of this information is not necessarily available for each and every pathology report.13 Of this information, tumor size, tumor type, histopathologic grade of tumor, lymph node involvement, and lymphovascular invasion are considered the most predictive prognostic factors.3
Anatomical Site: This describes the location where the sample was taken. For example: “breast, right, mastectomy” or “right breast, upper outer quadrant, lumpectomy.”13
Size: The exact size of the tumor is reported in centimeters.6,13 In general, the smaller the tumor the better the prognosis, and the larger the tumor, the poorer the prognosis. For this reason, the exact size of the primary tumor is considered an independent prognostic factor for disease recurrence. Tumors less than 2 centimeters generally have the best prognosis, and tumors greater than 5 centimeters have the worst prognosis.6
Type: Breast cancers are classified as either invasive (infiltrating) or noninvasive (in situ).1,6,9,13-18 Infiltrating carcinomas have crossed the basement membrane and have invaded the breast stroma. Such tumors have the potential to metastasize. In situ carcinomas have not crossed the basement membrane and show no evidence of microscopic invasion into the surrounding stroma.1-3,11-14
Invasive breast cancers are usually epithelial tumors of ductal or lobular origin.9 (Breast tissue is primarily made up of lobules that produce milk and ducts that carry the milk to the nipple.13) Infiltrating ductal carcinomas are the most common type of invasive breast cancer, accounting for 65% to 85% of all cases.1,6,9,15 Infiltrating lobular carcinomas account for 5% to 10% of invasive breast cancers.1,6,9,17 Other invasive histologies of epithelial origin, such as medullary, mucinous, tubular, and nonepithelial breast tumors (e.g., breast lymphoma) are much less common and together account for less than 10% of all invasive breast cancers.9
There are two subtypes of noninvasive carcinomas, ductal carcinoma in situ (DCIS) and lobular carcinoma in situ (LCIS).6,9,14,16,18 DCIS is the most common type of noninvasive breast cancer, accounting for 25% of all breast cancers, and can also be seen in association with invasive forms. The diagnosis of DCIS is increasing as more women are receiving regular mammograms, as well as due to advancements in mammography technology. If untreated, approximately 30% of women with DCIS will develop invasive breast cancer within 10 years of the initial diagnosis.16 LCIS is a microscopic diagnosis because there is no palpable mass and no specific clinical abnormality. Unlike DCIS, LCIS is usually undetectable by mammography. Consequently, the diagnosis of LCIS is usually a finding in biopsy specimens obtained because of symptoms or mammography findings consistent with benign lesions.6 About 25% of women who have LCIS develop invasive breast cancer within 25 years of the initial diagnosis.18
Histopathologic Grade: Infiltrating breast cancers are usually assessed by a pathologist with a histopathologic grade, which takes into account three parameters—nuclear grade, mitotic rate, and cellular differentiation. Each of these parameters is given a score of 1 to 3, resulting in a total score ranging from 3 to 9.1,13 Higher-grade tumors are associated with higher rates of distant metastasis and poorer survival.6
Nuclear grade describes the aggressiveness of the tumor. Grade 1 tumors have relatively uniform nuclei, whereas grade 3 tumors have nuclei that are atypical (vary in size and shape). Grade 2 tumors show features between 1 and 3.1,13 The higher the nuclear grade, the more aggressive the tumor.13
Mitotic rate indicates the number of malignant cells that are actively dividing. Grade 1 tumors are slowly dividing (with little or no evidence of mitotic activity), grade 2 tumors have a moderate mitotic rate, and grade 3 tumors have considerable mitotic activity.1,13,15,17 The higher the mitotic rate, the more aggressive the tumor.13
Cellular differentiation measures how closely the specimen resembles normal breast tissue. A grade of 1 represents well-differentiated tumor cells, a grade of 2 represents moderately differentiated tumor cells, and a grade of 3 represents poorly differentiated tumor cells.1,6,13 Higher cellular differentiation scores are associated with increased rates of distant metastasis and a poorer survival.6
Surgical Margins: When the pathology department receives a breast cancer specimen, the edges or borders are marked with ink. This enables the pathologist to determine whether the tumor goes right up to the inked border (positive) or whether the margin is clear of tumor (negative). Positive margins suggest that disease remains and usually indicates the need for additional surgical procedures to remove any remaining cancer and achieve negative margins.13
Lymph Node Status: An essential component of breast cancer surgery is axillary lymph node assessment.9 This is because the number of affected axillary lymph nodes is directly related to disease recurrence.6 If cancer is found in any of the lymph nodes, it is reported as node-positive. The pathology report indicates how many lymph nodes were removed and how many were positive (e.g., 0/12 is no positive lymph nodes out of 12; 1/13 is one positive lymph node out of 13).13
There are two methods currently in use for assessing axillary lymph nodes: sentinel node biopsy and axillary node dissection.9 Patients at low risk for having positive axillary lymph node involvement (e.g., low histopathologic grade, no evidence of palpable lymph nodes) commonly have a sentinel node biopsy first. This technique involves injecting blue dye and/or a radioactive material around the site of the tumor and allowing it to travel into the draining lymph nodes. The initial draining lymph node(s) are subsequently removed.9,13 Patients who are sentinel lymph node negative are much less likely to have positive axillary lymph nodes, compared with those who are sentinel node positive. The finding of a positive sentinel lymph node is usually followed by a standard axillary node dissection or surgical removal of more nodes.13
Lymphovascular Invasion: Lymphatic and vascular invasion, defined as evidence of tumor emboli in lymphatic and vascular spaces, has emerged in recent years as having prognostic significance for the risk of recurrence. This factor helps to identify node-negative patients who are at increased risk for lymph node involvement and metastases.6
Hormone Receptor Status: Hormone biomarker tests will appear on the pathology report. The predictive value of hormone receptors is well established; however, the prognostic value in regard to clinical outcomes is controversial.6,10 The hormone receptors clinically useful in discussions of breast cancer include the estrogen receptor (ER) and the progesterone receptor (PR).6 Tumors that have a high proportion of ER or PR are classified as hormone positive.6,13 Hormone-positive tumors respond to hormonal therapies up to 80% of the time, whereas hormone-negative tumors rarely respond to hormonal manipulation. This leaves patients who have hormone-negative tumors limited treatment options, with chemotherapy being utilized. The response rate in patients with ER-positive and PR-negative, or ER-negative and PR-positive, tumors is somewhere in between, but significant benefits may still be gained with hormonal therapy in these patients.6
Antiestrogen or endocrine therapy is the cornerstone of treatment for patients who have hormone-positive tumors. When used in the adjuvant (i.e., curative) setting, such therapies are typically given for 5 years, although the optimal duration of therapy is still under investigation.9,19 Commonly used therapies include selective estrogen receptor modulators (SERMs) such as tamoxifen and the aromatase inhibitors such as anastrozole, letrozole, and exemestane.6,9 Tamoxifen has long been considered the gold-standard treatment for hormone-positive premenopausal women.6,20 Due to improved outcomes, the aromatase inhibitors have replaced tamoxifen for hormone-positive postmenopausal women. The aromatase inhibitors can also be used in hormone-positive premenopausal women who have undergone ovarian ablation (surgically or pharmaceutically). Some data suggest that premenopausal patients who switch to the aromatase inhibitors (from tamoxifen) after they become postmenopausal have longer disease-free survival.6
Ki-67 Proliferation Index: The Ki-67, a cell proliferated associated nuclear antigen, is found in nearly all stages of the cell cycle and is therefore a direct indicator of the growth fraction. The Ki-67 status is reported as a percentage of invasive carcinoma cells exhibiting positive nuclear staining: less than 10% (favorable prognosis), more than 20% (unfavorable diagnosis), and 10% to 20% (borderline category). The Ki-67 growth fraction is significantly related to the grade in most tumors, being the highest in grade 3 invasive carcinomas. ER- and PR-negative tumors tend to have a high Ki-67 proliferation index. Notably, the Ki-67 proliferation index does not correlate well with the stage of the tumor as reflected in size, lymph node status, or distant metastases.13
HER2/neu Status: Gene overexpression of the human epidermal growth factor HER2/neu occurs in approximately 20% to 30% of breast cancers and is associated with increased tumor aggressiveness, increased rates of recurrence, and shortened survival.3-6,8,9 Depending upon the test used to measure HER2/neu gene overexpression, results are reported as positive, equivocal, or negative, or as 0, 1+, 2+, or 3+ (with 3+ having high levels of overexpression).6,8,13 While HER2/neu gene overexpression has traditionally been considered a poor prognostic factor, the outcome of patients with HER2/neu-positive breast cancer may be changing with the advent of targeted therapies, such as the anti-HER2/neu monoclonal antibody trastuzumab, and the oral tyrosine kinase inhibitor lapatinib, which are significantly improving patient outcomes.6,9,19,20
Interestingly enough, an American Society of Clinical Oncology expert panel on tumor markers in breast cancer did not recommend the use of HER2/neu for determining prognosis, largely because outcomes are heavily influenced by subsequent therapy.3
Gene Expression Profiling
Gene expression (molecular) profiling is also being use to provide prognostic and predictive information on the clinical outcomes of breast cancer. The Oncotype DX assay uses a reverse transcription-polymerase chain reaction assay of 21 genes to predict the likelihood of disease recurrence in lymph node-negative and ER-positive breast cancer while on endocrine therapy. A recurrence score is calculated with the Oncotype DX assay that categorizes patients into low-, intermediate-, or high-risk groups. A low recurrence score indicates that perhaps adjuvant chemotherapy could be avoided and endocrine therapy alone may be sufficient. A high recurrence indicates the need for endocrine therapy and chemotherapy. MammaPrint is another molecular prognostic test that uses DNA microarray analysis to measure the activity of a set of 70 genes to determine the likelihood of breast cancer recurrence in women with stage I or II breast cancer, tumor size 5 cm or less, and no lymph node involvement. To predict the risk of breast cancer metastases, MammaPrint uses an algorithm to issue a score that indicates whether the patient is at low or high risk.6
Despite the usefulness of these tests, the routine pathologic evaluation of a breast cancer must never be compromised by the demand to submit portions of the tumor for genetic profiling. This phenomenon is becoming increasingly problematic as the size of breast cancers decreases and the number of new assays increases.7
Staging and Prognosis
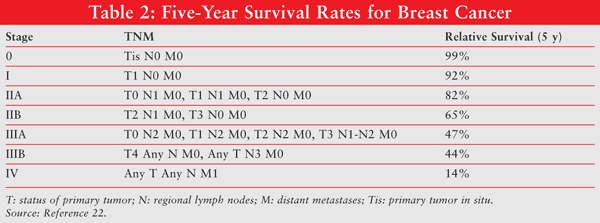
The prognosis for breast cancer generally depends on its stage. Certain information contained in the pathology report (e.g., tumor size, lymph node status) is used in the staging of breast cancer. The standard staging system for breast cancer, which was developed by the American Joint Committee on Cancer, is based on the size of the primary tumor (T), lymph node status (N), and whether there is evidence of metastatic disease (M). TABLE 1 further defines the TNM system for staging breast cancer.6,12,21The 5-year survival rates for the five stages of breast cancer based on their TNM staging are listed in TABLE 2.22

Uniqueness of Triple-Negative Breast Cancer
Triple-negative breast cancer refers to a specific subtype of breast cancer that is ER-negative, PR-negative, and HER2/neu-negative (hence the term triple-negative).20,23Clearly, the pathologic report is not only useful but necessary when diagnosing patients with triple-negative breast cancer.3
Because triple-negative breast cancers are hormone-negative and HER2/neu negative, they do not respond to endocrine therapy or trastuzumab, respectively. Generally speaking, triple-negative breast cancer is more aggressive, is associated with a poorer overall patient prognosis, and has only limited treatment options (i.e., chemotherapy alone is the only treatment option). Patients with triple-negative breast cancer have an increased likelihood of distant recurrence and of death compared with women who have nontriple-negative tumors. Triple-negative breast cancer is more common in younger women, in women with the BRCA1 mutation, and in women who are African American or Hispanic.20
Role of the Pharmacist
Much progress has been achieved in the management of breast cancer over the last decade. Women diagnosed with breast cancer today have a higher likelihood of surviving their cancers compared with women diagnosed in the 1970s and 1980s. This is most likely due to more effective screening. Because the curability of breast cancer is very much correlated to stage of diagnosis, it is essential for clinicians to be vigilant about screening and early detection.9 Clearly, it is important for pharmacists to understand the various components of the pathology exam and their impact on disease management so that they can provide exemplary pharmaceutical care to their patients living with breast cancer
The author acknowledges that she wrote this paper to educate pharmacists about a topic she was not familiar with until her own diagnosis with breast cancer in December 2007.
REFERENCES
1. Bleisweiss I. Pathology of breast cancer: the invasive carcinomas. In: Hayes D, ed. UpToDate. Waltham, MA: UpToDate; 2008.2. Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathological characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol.2008;26:4282-4288.
3. Hayes D. Measurement of prognostic factors in breast cancer. In: Schnipper L, ed. UpToDate. Waltham, MA: UpToDate; 2008.
4. Abeloff M, Wolff A, Weber B, et al. Cancer of the breast. In: Abeloff M, Armitage J, Niederhuber J, et al, eds. Abeloff’s Clinical Oncology. 4th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2008:2121-2153.
5. Fischgrabe J, Wulfing P. Targeted therapies in breast cancer: established drugs and recent developments. Curr Clin Pharmacol. 2008;3:85-98.
6. Michaud L, Espirito J, Esteva F. Breast cancer. In: DiPiro J, Talbert R, Yee G, et al, eds. Pharmacotherapy. 7th ed. New York, NY: McGraw-Hill; 2008:2121-2153.
7. Costanza M, Chen W. Epidemiology and risk factors for breast cancer. In: Hayes D, ed. UpToDate. Waltham, MA: UpToDate; 2008.
8. Yamauchi H, Hayes D. HER2 and predicting response to therapy in breast cancer. In: Schnipper L, ed. UpToDate. Waltham, MA: UpToDate; 2008.
9. Meisner AL, Fekrazad MH, Royce ME. Breast disease: benign and malignant. Med Clin North Am. 2008;92:1115-1141.
10. Breast cancer treatment (PDQ) Health Professional Version. National Cancer Institute. www.cancer.gov/cancertopics/
11. Genetic testing for BRCA1 and BRCA2: it’s your choice. National Cancer Institute Fact Sheet. www.cancer.gov/cancertopics/
12. Detailed guide: breast cancer. Can breast cancer be found early? American Cancer Society. www.cancer.org/docroot/CRI/
13. Understanding your breast cancer pathology report: a guide for breast cancer patients. National Breast Cancer Organization. www.networkofstrength.org/
14. Bleisweiss I. Pathology of breast cancer: the in situ carcinomas. In: Hayes D, ed. UpToDate. Waltham, MA: UpToDate; 2008.
15. Breast cancer: invasive ductal carcinoma. College of American Pathologists. www.cap.org/apps/portlets/
16. Breast cancer: ductal carcinoma in situ. College of American Pathologists. www.cap.org/apps/docs/
17. Breast cancer: invasive lobular carcinoma. College of American Pathologists. www.cap.org/apps/docs/
18. Breast condition: lobular neoplasia (lobular carcinoma) in situ. College of American Pathologists. www.cap.org/apps/docs/
19. Bedard PL, Cardoso F. Recent advances in adjuvant systemic therapy for early-stage breast cancer. Ann Oncol. 2008;19(suppl 5):v122-v127.
20. Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429-4434.
21. TNM staging classification for breast cancer. In: Hayes D, ed. UpToDate. Waltham, MA: UpToDate; 2008.
22. Breast cancer, staging and prognosis, treatments, and treatment results. Cancer Monthly. www.cancermonthly.com/cancer_
23. Winer E, Mayer E. Optimizing treatment of “triple-negative” breast cancer. Presented at: 30th Annual San Antonio Breast Cancer Symposium; December 13-16, 2007; San Antonio, TX.
To comment on this article, contact rdavidson@jobson.com.





