US Pharm. 2006;5:HS-2-HS-16.
Clonidine was originally
developed in 1962 for use as a nasal decongestant.1 However, rapid
recognition of its ability to reduce blood pressure led to the FDA approval of
this agent for the treatment of hypertension in 1974.2 During the
1970s, clonidine gained popularity as treatment for hypertension since it was
not linked with the postural and exercise-induced hypotension common in other
antihypertensive regimens. However, unwanted side effects of drowsiness, dry
mouth, and sympathetic overactivity upon abrupt discontinuation led to a
decline in its use. In 1996, a transdermal formulation renewed interest in the
drug, as reported side effects were less pronounced than with oral treatment.
Today, with the development
and marketing of newer products, the use of clonidine in the treatment of
hypertension is limited; however this agent's ability to modify both central
and peripheral adrenergic transmission is proving to be of increasing interest
to health care practitioners. Clonidine's unique mechanism of action has
prompted many to investigate its therapeutic potential in several different
disease states.
DOSAGE FORMS
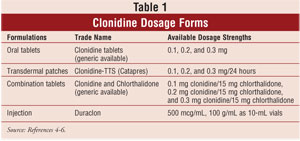
Clonidine is available as an oral
tablet form, a transdermal therapeutic system (Catapres-TTS), and as an
injection for epidural use (Duraclon) (Table 1). The oral and topical
formulations are FDA approved only for the treatment of hypertension, while
the injectable form is FDA approved only for intractable cancer pain, in
combination with opioids.3-6
ADVERSE EFFECTS
Most of the
common adverse effects with oral clonidine are mild and tend to diminish with
continued treatment or with a reduction in dosage. The most frequent adverse
effects seen with oral and transdermal clonidine therapy are dry mouth and
drowsiness; however, dizziness, sedation, constipation, and headache are also
common reactions noted. Systemic effects with transdermal clonidine appear to
be less severe and may occur with less frequency than with oral therapy. Some
localized skin reactions have been reported with the transdermal system, with
the most common dermatologic reactions being localized pruritus and erythema.3-7
The injectable form of
clonidine has an adverse effect profile similar to both the oral and
transdermal forms. The most commonly reported adverse event during clinical
trials was a major decrease in blood pressure, particularly during the first
few days of therapy. Other common side effects include postural hypotension,
dry mouth, somnolence, dizziness, and confusion. Accidental dislodging of the
catheter can result in rebound hypertension secondary to abrupt withdrawal.3-7
DRUG INTERACTIONS
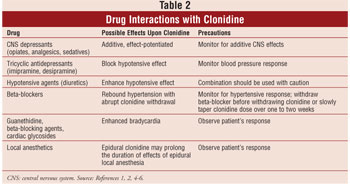
Clonidine is not
without its share of drug interactions (see Table 2). Of particular note is
the additive effect that is exhibited when combined with central nervous
system depressants and anesthetic agents. Tricyclic antidepressants have been
found to block the hypotensive effect of clonidine, whereas diuretics and
other hypertension agents may enhance the hypotensive effect. Furthermore,
clinicians should be aware that beta-adrenergic antagonists might exacerbate
hypotension upon withdrawal of clonidine.3-7
THERAPEUTIC USES
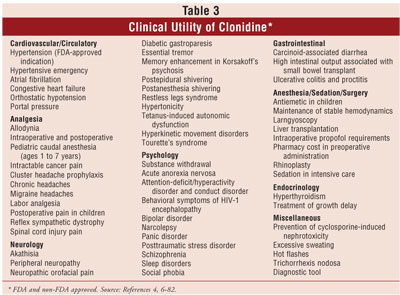
The clinical
utility of clonidine has been reported in many clinical trials and case
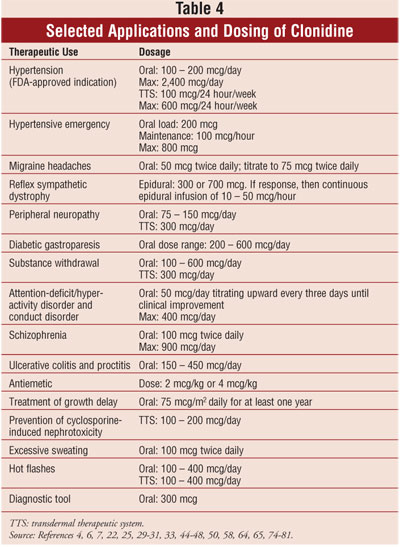
reports (table 3). A representative study or report highlights each
therapeutic use (Table 4). Notably, there may be more citations published for
a given therapeutic use than referenced in this article. The only FDA-approved
indication for clonidine is for the treatment of hypertension, and when given
epidurally, for cancer pain relief; all other uses described are off-label.
Cardiovascular/Circulatory
Hypertension:4,6
The recommended regimen is 100 to 200 mcg/day in two divided doses, with a
maximum of 2,400 mcg/day. Dosage for the transdermal therapeutic system is 100
mcg per 24 hours every week, with a maximum of 600 mcg per 24 hours every
week. Doses up to 5,000 mcg/day have been attempted with unwanted side effects
and no additional efficacy.
Hypertensive Emergency:
Karachalios7 studied 38 severely hypertensive patients (systolic
175 to 210 mmHg, diastolic 105 to 130 mmHg). After observing patients at rest
for one to two hours, an oral loading dose of clonidine 200 mcg was given,
followed by 100 mcg/hour, to a maximum total dose of 800 mcg. Thirty-five
patients responded well with a decreased mean blood pressure in six hours of
145 ± 20 mmHg systolic and 98 ± 15 mmHg diastolic. Side effects were minimal.
Atrial Fibrillation:
In 1992, Roth et al.8 determined that low-dose clonidine was
effective in slowing ventricular rate in patients with rapid atrial
fibrillation. In 2001, Simpson et al.9 used clonidine to treat
atrial fibrillation in 40 patients with new-onset, stable, rapid atrial
fibrillation. Thus, clonidine controls ventricular rate with an efficacy
comparable to that of digoxin and verapamil.
Congestive Heart
Failure (CHF): Manmontri
and MacLeod10 studied clonidine in CHF treatment. Clonidine 200 to
400 mcg/day orally significantly reduced heart rate and increased left
ventricular ejection fraction, as well as improved New York Heart Association
functional status. However, defects in the design require more study before
clonidine can be recommended. Azevedo et al.11 studied the effect
of intravenous (IV) clonidine on cardiac sympathetic activity and left
ventricular function in CHF patients. Nine patients received a 50- or 100-mcg
bolus of IV clonidine. The authors concluded that use of clonidine in CHF
warrants further exploration.
Orthostatic
Hypotension (OH):
Stumpf and Mitrzyk12 conducted a clinical review of treatment
options for OH. Oral clonidine was titrated in patients with refractory OH.
Patients given doses of 400 and 800 mcg showed improvement in standing time
and blood pressure. Dry mouth and sedation were reported as the major side
effects; however, these disappeared with long-term use.
Portal Pressure:
Lin et al.13 studied the effect of combining propranolol with
clonidine in patients with posthepatitic cirrhosis. Twenty patients received
an IV injection of 0.1 mg/kg propranolol, followed 30 minutes later by 150 mcg
of clonidine. Researchers concluded that the reduction in mean arterial
pressure might limit the clinical utility of this combination in patients with
already low arterial pressure.
Analgesia
Allodynia:
Allodynia is a condition of sympathetically maintained pain, with ongoing pain
and hyperalgesia.14 Davis et al. examined the use of topical
clonidine patches to treat the hyperalgesia. Clonidine 200 and 300 mcg patches
were applied to affected areas; each patch was left in place for two to 10
days. Topical clonidine significantly reduced hyperalgesia.14 Olson
et al.15 reported on a patient with an electrical injury who was
treated with topical clonidine. Eisenach et al.16 studied the use
of intrathecal and IV clonidine to treat hyperalgesia from intradermal
capsaicin injection in volunteers. Volunteers were randomized into four
treatment groups: IV 50-mcg or 150-mcg injections and intrathecal 50-mcg or
150-mcg injections. Both doses of IV injections and the lower dose intrathecal
administration did not produce any relief, whereas the 150-mcg intrathecal
dose lessened pain within 45 minutes, with effects lasting at least four
hours.
Intraoperative and
Postoperative: De
Kock et al.17 compared epidural clonidine to sufentanil in the
perioperative period. Patients received epidural clonidine or sufentanil for
12 hours. Clonidine improved intraoperative hemodynamic stability and provided
the same amount of postoperative analgesia as did sufentanil. Yet, clonidine
had a longer-lasting residual analgesic effect, thus decreasing postoperative
analgesic demands.
Pediatric Caudal
Anesthesia (ages 1 to 7 years):
Jamali et al.18 studied pediatric patients ages 1 to 7 years who
received 1 mcg/kg of clonidine, 1/200,000 of epinephrine, or control in
addition to 1 mL/kg of 0.25% bupivacaine. The duration of postoperative
analgesia was significantly increased in the clonidine group, compared to
bupivacaine alone or bupivacaine with epinephrine. Fewer clonidine patients
required postoperative analgesia.
Intractable Cancer
Pain: Eisenach et
al.19 conducted a double-blind study in patients unresponsive to
morphine who received 30 mcg/hour of epidural clonidine or placebo for 14
days. Pain was assessed using visual analog score and daily epidural morphine
use. Patients with neuropathic pain gained the best relief from clonidine,
with an overall success rate over placebo.
Cluster Headache
Prophylaxis: Leone
et al.20 studied transdermal clonidine in doses of 200 to 300
mcg/day. Five of 15 patients reported improvement during days 7 to 12 of
therapy. That the improvement was a result of reaching the final phase of the
cluster periods could not be ruled out. More studies are needed to clarify the
efficacy of transdermal clonidine to treat cluster headaches.
Chronic Headaches: Dalessio21
began therapy with transdermal clonidine 100 mcg/day for women under 130 lb
and 200 mcg/day for women more than 130 lb and for all men. After 18 weeks,
patients were switched to oral clonidine 100 to 200 mcg/day; dosage was
tapered off over three to five days. This protocol was successful in nine of
12 patients with chronic headaches.
Migraine Headaches: A
review by Wood22 suggests oral clonidine 50 mcg twice daily,
titrated to 75 mcg twice daily, was effective, with mild side effects.
Labor Analgesia: Gautier
et al.23 randomized patients requiring labor analgesia to various
intrathecal solutions for analgesia, consisting of clonidine and sufentanil.
Clonidine injection 30 mcg combined with intrathecal sufentanil significantly
increased the duration of analgesia during the first stage of labor without
adverse maternal or fetal effects.
Postoperative Pain in
Children: Mikawa et
al.24 examined clonidine in children undergoing minor surgery.
Ninety children ages 5 to 12 years received placebo or clonidine (2 or 4
mcg/kg) 105 minutes before anesthesia induction. Clonidine 4 mcg/kg provided
more postoperative analgesia than did the other treatment groups.
Reflex Sympathetic
Dystrophy: Rauck et
al.25 randomized 26 patients with chronic pain to 300 or 700 mcg of
epidural clonidine or placebo. Responders then received a continuous epidural
infusion of 10 to 50 mcg/hour of clonidine. Clonidine was shown to provide
pain relief.
Spinal
Cord Injury Pain: Siddall
et al.26 reported on a patient who had received multiple agents to
treat spinal cord injury pain for one year. Intrathecal clonidine 17 mcg/day
combined with morphine 10 mg/day produced a 50% reduction in previous pain
levels.
Neurology
Akathisia: Blaisdell27
referenced a study with six patients who showed major improvement, four of
whom obtained complete remission. Adler et al.28 titrated
oral clonidine to a maximum of 150 to 400 mcg/day and observed significantly
reduced akathisia and anxiety at the maximum dose. Sedation was a problematic
side effect.
Peripheral Neuropathy:
Tan and Croese29 reported cases where excellent pain relief of
disabling leg pain was obtained using oral clonidine 75 to 100 mcg/day.
Schwartz and Rosenfeld30 reported a case in which oral clonidine 75
mcg twice daily was used with rapid and complete relief of painful muscle
cramps and pruritus. Kingery31 reported that transdermal clonidine
at doses of 300 mcg daily had little effect on pain, yet a subset of patients
did have analgesia. Oral clonidine at doses of 200 mcg/day provided pain
relief.
Neuropathic Orofacial
Pain: Epstein et al.32
examined 17 patients who applied clonidine in a cream (200 mcg/g) four times
daily to the site of pain. Improvement was noticed in patients diagnosed with
both neuropathic pain (50% of patients) and neuralgia (67% of patients).
Diabetic Gastroparesis:
Rosa-e-Silva et al.33 evaluated the effects of clonidine in
patients with longstanding diabetes and evidence of autonomic neuropathy.
Treatment with oral clonidine was given in doses ranging from 200 to 600
mcg/day. Results showed that gastric emptying half-time and symptoms decreased
in all patients.
Essential Tremor:
Koller et al.34 evaluated clonidine in 10 patients randomized to
receive either placebo or incremental doses of clonidine until a dose of 600
mcg was reached or unwanted side effects occurred. Neither placebo nor
clonidine significantly reduced hand tremor.
Memory Enhancement in
Korsakoff's Psychosis: McEntee
and Mair35 studied patients with chronic memory disorder to assess
if clonidine, d-amphetamine, or methysergide would improve memory. Clonidine
was linked with major memory improvement.
Postepidural Shivering:
Yang et al.36
studied 40 patients who were randomly assigned to receive either normal saline
or clonidine 150 mcg diluted in 10 mL of normal saline 20 minutes before
epidural administration. Other studies suggest that shivering occurs in 30% to
64% of people receiving epidural lidocaine. This study showed that the
incidence of shivering in the clonidine group was 5%, and clonidine was
effective in preventing shivering associated with epidural anesthesia.
Postanesthesia
Shivering: Generali
and Cada37 conducted a literature review of 295 patients who
received a single clonidine dose of 3 mcg/kg at various times throughout the
surgical procedure. Single-dose clonidine 3 mcg/kg was as effective as
meperidine and superior to placebo in the prevention of postanesthetic
shivering.
Restless Legs Syndrome:
Zoe et al.38 reported a case that was resistant to traditional
therapy. The patient was then treated with clonidine 100 mcg at bedtime,
titrated to 900 mcg/day. After 15 months of successful treatment, the dosage
was reduced to 450 mcg/day due to the reported side effect of dry mouth.
Hypertonicity:
Donovan et al.39 studied the use of clonidine in 55 patients with
spasticity after sustaining a spinal cord injury. Over half (56%) of patients
treated with oral clonidine 100 to 400 mcg daily showed some relief.
Tetanus-Induced
Autonomic Dysfunction: Sutton
et al.40 reported the case of a man sustaining a compound fracture
in which tetanus had set in. After inadequate response to magnesium sulfate,
clonidine 300 mcg every eight hours was added to control sympathetic
overactivity. Clonidine dramatically improved cardiovascular instability and
allowed reduction of muscle relaxants and sedation.
Hyperkinetic Movement
Disorders: Two
reviews41,42 suggest that oral clonidine, titrated to a range of
100 to 800 mcg/day, may relieve symptoms in children. Phonic tics were more
responsive to clonidine. Main adverse effects included sedation, dry mouth,
and potential rebound hypertension upon withdrawal.
Tourette's Syndrome:
Leckman et al.43
randomized 47 patients to receive clonidine 4 to 5 mcg/kg to a maximum of 250
mcg/day or placebo. Clonidine reduced symptoms of impulsivity, hyperactivity,
and tics associated with Tourette's syndrome.
Psychology
Substance
Withdrawal: Robinson
et al.44 examined the effectiveness of clonidine in the treatment
of alcohol withdrawal. The study was double-blind, and patients were
randomized to receive either clonidine or chlormethiazole. Clonidine was found
less effective and had greater serious adverse effects than chlormethiazole.
Keshavan et al.45 used oral clonidine 600 mcg/day to treat a
patient undergoing benzodiazepine withdrawal. After previous failure of a
medically supervised taper of lorazepam, the addition of clonidine allowed
lorazepam to be tapered over two weeks without any withdrawal symptoms. McGee
and Murray46 reviewed four cases in which clonidine had been used
to treat nicotine withdrawal. Doses of 100 to 300 mcg/day were useful adjuncts
to a cessation program. Clonidine helped relieve patients' anxiety related
symptoms. Cheskin et al.47 compared clonidine to buprenorphine for
the treatment of acute opioid detoxification. Patients were randomly assigned
to receive either buprenorphine or clonidine. Buprenorphine provided greater
relief of withdrawal symptoms and had fewer side effects than clonidine. Sovner48
reported two cases of patients who were experiencing thioridazine
withdrawal–induced behavior. They were given oral clonidine 300 mcg twice
daily or transdermal clonidine 300 mcg/day. Clonidine enabled a successful
withdrawal from thioridazine, and successful tapering of clonidine dosage was
later achieved.
Acute Anorexia Nervosa:
Casper et al.49 studied four treatment-resistant patients in a
placebo-controlled crossover trial. Clonidine was initiated at 150 mcg/day and
was gradually increased to a maximum of 500 to 700 mcg/day. Clonidine was not
superior to placebo for promoting weight gain, nor did clonidine intensify the
urge to eat before and after meals.
Attention-Deficit/Hyperactivity
Disorder (ADHD) and Conduct Disorder:
Schvehla et al.50 conducted a retrospective study of 18
prepubescent boys who received clonidine after failed trials of conventional
psychostimulants. Each child received oral clonidine 50 mcg/day, titrating
upward until clinical improvement or a maximum dose of 400 mcg was reached.
The average clonidine dose used was 8 mcg/kg. Eleven of the 18 children
exhibited marked clinical improvement. Sedation was the primary side effect,
lasting two to three days in 40% of patients following dosage increase.
Behavioral Symptoms of
HIV-1 Encephalopathy:
Ceseña et al.51 reported the case of a four-year-old boy
with AIDS who exhibited hyperactivity and impulsive behavior. The patient was
given oral clonidine 25 mcg/day, which was slowly increased to 25 mcg three
times daily. Improvement was seen in hyperactivity, impulsivity, and sleeping
patterns, and aggressive behavior decreased.
Bipolar Disorder:
Janicak et al.52 studied clonidine in the acute mania phase of
bipolar disorder. Twenty-one patients received placebo or clonidine for two
weeks. The average maximum daily dose of clonidine was 470 mcg. Clonidine was
no more effective than placebo, and side effects contributed to a
significantly higher dropout rate. Kontaxakis et al.53 reported a
case in which oral clonidine was used in the treatment of refractory mixed
bipolar disorder. Oral clonidine 125 mcg twice daily was added to the
patient's regimen. Within two days, the patient had rapid improvement in
psychopathology, followed by a decline in effect. The dose was eventually
titrated slowly to 600 mcg/day with no significant side effects noted.
Bakchine et al.54 reported a case of a woman with focal brain
damage in a manic-like state who was given alternating trials of clonidine 300
mcg twice daily, placebo tablets, carbamazepine, and then
levodopa-benserazide. Clonidine produced a marked decrease in manic symptoms
and improved cognitive functions.
Narcolepsy:
Salín-Pascual et al.55 studied the effects of clonidine on two
patients given 150 mcg/m2 in the morning. One week later, they were
given placebo, followed by clonidine on two consecutive nights. Following the
blind trial, patients were then given oral clonidine 225 mcg at bedtime, and
recordings were taken at predefined intervals. A rapid improvement was seen in
both patients receiving clonidine. Sleep attacks, daytime sleepiness, and
cataplexy disappeared.
Panic Disorder: Puzantian
and Hart56 cited four studies demonstrating that oral clonidine in
doses of 100 mcg twice daily to 2 mg/day should be considered as a last-line
anxiolytic agent. However, no clinical indicators of success were identified.
Posttraumatic Stress
Disorder: Kinzie
and Leung57 examined patients who received 50 mg of imipramine at
night, which was increased to 150 mg over three weeks. If there was no
response after three weeks, clonidine 100 mcg twice daily was added. Results
showed that combined with imipramine 150 mg/day, all patients receiving
clonidine symptomatically improved.
Schizophrenia:
Freedman et al.58 compared clonidine to a neuroleptic and placebo
in eight patients with schizophrenia. Oral clonidine was dosed initially at
100 mcg twice daily and increased to 900 mcg/day. Results suggested that
clonidine and neuroleptics may be equally efficacious as antipsychotics in
treating schizophrenia. Clonidine reduced symptoms of psychosis resulting from
withdrawal of drugs that have produced tardive dyskinesia.
Sleep Disorders: Horacek59
used clonidine extemporaneously compounded extended-release capsules for sleep
disorders. The combination of immediate-release oral clonidine one hour before
bedtime with extended-release clonidine several hours before bedtime helped
prevent sleep disorders. The immediate-release capsule aids in onset of sleep,
while the extended release prevents rebound hyperarousal during the night. The
extended-release capsules were compounded to release clonidine over an
eight-hour period. Rubinstein et al.60 reported the case of a child
with ADHD who had chronic sleep problems due to the use of dextroamphetamine.
Oral clonidine 50 mcg was started 45 minutes prior to sleep. Clonidine had an
almost immediate impact in the child's sleep pattern, which greatly improved
over a two-month period.
Social Phobia: Goldstein61
reported the case of a patient with increased discomfort and anxiety in social
situations. After several trials with other agents, clonidine 100 mcg twice
daily was started. After one week, the patient had a remarkable decrease in
frequency and intensity of panic attacks. After four months, the patient had
only infrequent attacks. No side effects were reported.
Gastrointestinal
Carcinoid-Associated
Diarrhea: Schwörer
et al.62 reported a case in which a man with carcinoid syndrome
treated with octreotide and interferon developed diarrhea (six to eight watery
stools a day). The patient was treated with oral clonidine 75 mcg three times
a day and 150 mcg at bedtime. This reduced stool frequency to two to three
stools per day, with no defecation at night. Mild sedation and dry mouth were
noted.
High Intestinal Output
Associated with Small Bowel Transplant: Rovera
et al.63 conducted a study of 13 patients who underwent small bowel
transplant to assess the efficacy of clonidine in reducing intestinal output.
Oral clonidine was initiated at doses of 25 mcg twice daily in children and 50
mcg twice daily in adults. Loperamide, tincture of opium, paregoric, diphen
oxylate/atropine, and somatostatin were used alone or in combination for
control of output. Results showed that intestinal output was unchanged in
children; however, adult output decreased by a mean of 700 mL.
Ulcerative Colitis and
Proctitis: Melander
et al.64 reported seven cases in which oral clonidine was used to
treat ulcerative colitis or proctitis. At doses of 150 to 450 mcg daily, some
patients temporarily experienced diminished and more solid stools. Adverse
effects of fatigue and nausea seemed to outweigh the possible benefits of
clonidine.
Anesthesia/Sedation/Surgery
Antiemetic
in Children: Mikawa
et al.65 conducted a trial to determine if preoperatively
administered oral clonidine reduced the incidence of vomiting in children
following strabismus surgery. One hundred forty children (ages 3 to 12 years)
were randomized to placebo, diazepam 0.4 mcg/kg, clonidine 2 mcg/kg, or
clonidine 4 mcg/kg in 2 mL/kg of apple juice. Agents were administered 100
minutes prior to induction of anesthesia. The incidence and frequency of
vomiting was found to be lower in the clonidine 4 mcg/kg group.
Maintenance of Stable
Hemodynamics: Costello
and Cormack66 compared clonidine to temazepam in controlling
hemodynamics during pin head-holder application during a craniotomy. Fifty
patients took oral clonidine 3 mcg/kg or oral temazepam 10 to 20 mg given 90
minutes before induction of anesthesia. Clonidine was effective in reducing
mean arterial blood pressure increases that result from pin head-holder
application.
Laryngoscopy:
Laurito et al.67 studied the effects of oral clonidine
premedication on sedation, and hemodynamic responses during preoperative
period, laryngoscopy, and postanesthesia recovery. Patients took clonidine 100
mcg, clonidine 200 mcg, triazolam 0.25 mg, or placebo. Oral clonidine 200 mcg
given 90 minutes prior to anesthetic induction effectively sedated and blunted
the hemodynamic response, but anxiolytic effects were not seen.
Liver Transplantation: De
Kock et al.68 examined the effectiveness of clonidine on fluid
requirements and hemodynamic stability during liver transplantation surgery.
Twenty patients were randomized to receive either slow IV bolus of clonidine 4
mcg/kg during anesthesia induction or control. IV clonidine significantly
reduced the need for IV fluids and blood products and did not compromise
circulatory stability.
Intraoperative
Propofol Requirements:
Guglielminotti et al.69 studied 28 patients randomized in this
double-blind study to receive hydroxyzine 1 mg/kg or clonidine 5 mcg/kg two
hours before induction of anesthesia. Clonidine significantly reduced
intraoperative propofol requirements, compared to hydroxyzine, without adverse
effects on recovery or hemodynamic stability.
Pharmacy Cost in
Preoperative Administration:
Vallès et al.70 examined 80 patients randomly assigned to
receive 300 mcg of oral, intramuscular, or epidural clonidine or placebo at a
set time prior to surgery. Results indicated that in all groups premedicated
with clonidine, independent of route of administration, the expense of
isoflurane during anesthesia of about two hours' duration was significantly
reduced. Notably, the cost of epidural clonidine offset the savings in
isoflurane.
Rhinoplasty: Britto
et al.71 studied clonidine in 20 patients randomized to
receive temazepam 10 mg or temazepam 10 mg plus clonidine 3 mcg/kg 45 minutes
prior to induction of anesthesia. Clonidine with temazepam 10 mg was superior
to temazepam alone in decreasing mean arterial blood pressure, attenuating
response to intubation, and providing a better blood-free surgical field.
Sedation in Intensive
Care: Böhrer et al.72
reported on a patient who underwent distal esophagectomy and proximal
gastrectomy who was not achieving adequate sedation through the combination of
midazolam and fentanyl infusions. Clonidine 0.014 mcg/kg/min continuous
infusion was then added with excellent analgesia and sedation control.
Clonidine abrupt withdrawal led to circulatory problems requiring a 12-day
weaning period.
Endocrinology
Hyperthyroidism:
Herman et al.73
studied the effect of clonidine on inhibiting biological effects of
catecholamines released during hyperthyroidism. Patients with hyperthyroidism
received either nadolol 40 mg twice daily for one week or clonidine 150 mcg
twice daily for one week. Clonidine had similar clinical effects to nadolol.
Treatment of Growth
Delay: Moreno
Esteban et al.74 studied clonidine in 112 prepubescent children.
Oral clonidine 75 mcg/m2 was given daily for at least one year.
Clonidine may increase growth velocity in at least 65% of prepubescent
patients with constitutional growth delay.
Miscellaneous
Miscellaneous
uses have also been documented, including prevention of cyclosporine-induced
nephrotoxicity, excessive sweating, hot flashes, trichorrhexis nodosa, and as
a diagnostic tool for pheochromocytoma.18,75-83
CONCLUSION
The clinical
utility of clonidine has been demonstrated through many varied applications.
This article compiles case reports and studies to clarify dosage and outcomes
associated with each therapeutic use. The available literature on clonidine
and its clinical utility was reviewed. However, there may be more reports for
a given therapeutic use than cited in this article; original references should
be checked for more information before the drug is used for the listed disease
states. Many of these studies involved the use of oral clonidine, which
resulted in an undesirable outcome or a discontinuation of treatment due to
unwanted side effects. Sedation and dry mouth are more pronounced with oral
therapy and when doses are initiated at 200 mcg/day or greater. Initiating
therapy with doses of 100 mcg each night can minimize these adverse effects.
Due to the small number of patients in these studies, the findings cannot be
extrapolated to the general population. These factors should be considered
before applying these data to clinical practice. Although clonidine may have
limited use in the initial treatment of hypertension, this article evidences
its clinical utility in various disease states.
REFERENCES
1. Oesterheld J,
Tervo R. Clonidine: a practical guide for usage in children. South Dakota J
Med. 1996;234-237.
2. Mahoney A, Seeley
H. Clonidine: old friend–new guises. Br J Hosp Med. 1990;44:358-361.
3. Lowenstein J.
Drugs five years later: clonidine. Ann Intern Med. 1980;92:74-77.
4. Catapres
(clonidine) product information. Ridgefield, CT; Boehringer Ingelheim: 4/98.
5. Duraclon (epidural
clonidine) product information. Columbus, Ohio; Roxane Laboratories: 5/00.
6. Clonidine. In:
Facts and Comparisons. St. Louis, MO: Facts and Comparisons: 2004.
7. Karachalios GN.
Hypertensive emergencies treated with oral clonidine. Eur J Clin Pharmacol.
1986;31:227-229.
8. Roth A, Kaluski E,
Felner S, et al. Clonidine for patients with rapid atrial fibrillation. Ann
Intern Med. 1992;116:388-390.
9. Simpson CS, Ghali
WA, Sanfilippo AJ, et al. Clinical assessment of clonidine in the treatment of
new-onset rapid atrial fibrillation: a prospective, randomized clinical trial.
Am Heart J. 2001;142:e3.
10. Manmontri A,
MacLeod SM. Centrally acting sympatholytic agents in the treatment of
congestive heart failure. Drugs. 1990;40:169-175.
11. Azevedo ER,
Newton GE, Parker JD. Cardiac and systematic sympathetic activity in response
to clonidine in human heart failure. J Am Coll Cardiol. 1999;33:186-191.
12. Stumpf JL,
Mitrzyk B. Management of orthostatic hypotension. Am J Hosp Pharm.
1994;51:648-652.
13. Lin HC, Tsai YT,
Yang MC, et al. Haemodynamic effects of a combination of propranolol and
clonidine in patients with post-hepatitic cirrhosis. J Gastroenterol Hepatol.
1995;10:281-286.
14. Davis KD, Treede
RD, Raja SN, et al. Topical application of clonidine relieves hyperalgesia in
patients with sympathetically maintained pain. Pain. 1991;47:309-317.
15. Olson EE, Hogan
QH, Abram SE. Comments on topical clonidine for relief from allodynia. Pain.
1993;54:361.
16. Eisenach JC, Hood
DD, Curry R. Intrathecal, but not intravenous, clonidine reduces experimental
thermal or capsaicin-induced pain and hyperalgesia in normal volunteers.
Anesth Analg. 1998;87:591-596.
17. De Kock M,
Famenne F, Deckers G, Scholtes JL. Epidural clonidine or sufentanil for
intraoperative and postoperative analgesia. Anesth Analg. 1995;81:1154-1162.
18. Jamali S, Monin
S, Begon C, Dubousset AM. Clonidine in pediatric caudal anesthesia. Anesth
Analg. 1994;78:663-666.
19. Eisenach JC,
DuPen S, Dubois M, et al. Epidural clonidine analgesia for intractable cancer
pain. The Epidural Clonidine Study Group. Pain. 1995;61:391-399.
20. Leone M,
Attanasio A, Grazzi L, et al. Transdermal clonidine in the prophylaxis of
episodic cluster headache: an open study. Headache. 1997;37:559-560.
21. Dalessio DJ.
Clonidine in chronic headaches. Headache. 1991;31:257.
22. Wood RA. The
therapeutic uses of clonidine. Scott Med J. 1979;24:226-232.
23. Gautier PE, De
Kock M, Fanard L, et al. Intrathecal clonidine combined with sufentanil for
labor analgesia. Anesthesiololgy. 1998;88:651-656.
24. Mikawa K, Nishina
K, Maekawa N, Obara H. Oral clonidine premedication reduces postoperative pain
in children. Anesth Analg. 1996;82:225-230.
25. Rauck RL,
Eisenach JC, Jackson K, et al. Epidural clonidine treatment for refractory
reflex sympathetic dystrophy. Anesthesiology. 1993;79:1163-1169.
26. Siddall PJ, Gray
M, Rutkowski S, Cousins MJ. Intrathecal morphine and clonidine in the
management of spinal cord injury pain: a case report. Pain. 1994;59:147-148.
27. Blaisdell GD.
Akathisia: a comprehensive review and treatment summary. Pharmacopsychiat.
1994;27:139-146.
28. Adler LA, Angrist
B, Peselow E, et al. Clonidine in neuroleptic-induced akathisia. Am J
Psychiatry. 1987;144:235-236.
29. Tan YM, Croese J.
Clonidine and diabetic patients with leg pains. Ann Intern Med.
1986;105:633-634.
30. Schwartz J,
Rosenfeld V. Clonidine for painful diabetic-uremic leg cramps and pruritus–a
case report. Angiology. 1993;44:985.
31. Kingery, WS. A
critical review of controlled clinical trials for peripheral neuropathic pain
and complex regional pain syndromes. Pain. 1997;73:123-139.
32. Epstein JB,
Grushka M, Le N. Topical clonidine for orofacial pain: a pilot study. J Orofac
Pain. 1997;
11:4346-4352.
33. Rosa-e-Silva L,
Troncon LE, Oliveira RB, et al. Treatment of diabetic gastroparesis with oral
clonidine. Aliment Pharmacol Ther. 1995;9:179-183.
34. Koller W,
Herbster G, Cone S. Clonidine in the treatment of essential tremor. Movement
Disorders. 1986;1:235-237.
35. McEntee WJ, Mair
RG. Memory enhancement in Korsakoff's psychosis by clonidine: further evidence
for a noradrenergic deficit. Ann Neurol. 1980;7:466-470.
36. Yang CH, Yu CC,
Seah YS, et al. Effect of intravenous clonidine on prevention of postepidural
shivering. Acta Anaesthesiol Sin. 1993;31:121-126.
37. Generali J, Cada
DJ. Clonidine: postanesthesia shivering. Hosp Pharm. 2005;40:570-581.
38. Zoe A, Wagner ML,
Walters AS. High-dose clonidine in a case of restless legs syndrome. Ann
Pharmacother. 1994;28:878-881.
39. Donovan WH,
Carter RE, Rossi CD, Wilkerson MA. Clonidine effect on spasticity: a clinical
trial. Arch Phys Med Rehabil. 1988;69:193-194.
40. Sutton DN,
Tremlett MR, Woodcock TE, Nielsen MS. Management of autonomic dysfunction in
severe tetanus: the use of magnesium sulphate and clonidine. Intensive Care
Med. 1990;16:75-80.
41. Bressman, SB,
Greene PE. Treatment of hyperkinetic movement disorders. Neurol Clin.
1990;8:51-75.
42. Jankovic J.
Recent advances in the management of tics. Clin Neuropharmacology.
1986;9:S100-S110.
43. Leckman JF,
Hardin MT, Riddle MA, et al. Clonidine treatment of Gilles de la Tourette's
syndrome. Arch Gen Psychiatry. 1991;48:324-328.
44. Robinson BJ,
Robinson GM, Maling TJB, Johnson RH. Is clonidine useful in the treatment of
alcohol withdrawal. Alcohol Clin Exp Res. 1989;13:95-98.
45. Keshavan JS,
Crammer JL. Clonidine in benzodiazepine withdrawal. Lancet. 1985;1:1325-1326.
46. McGee KH, Murray
KM. Clonidine in nicotine withdrawal. DICP Ann Pharmacotherapy.
1989;23:473-474.
47. Cheskin LJ,
Fudala PJ, Johnson RE. A controlled comparison of buprenorphine and clonidine
for acute detoxification from opioids. Drug Alcohol Dependence.
1994;36:115-121.
48. Sovner R.
Thioridazine withdrawal-induced behavioral deterioration treated with
clonidine: two case reports. Mental Retardation. 1995;33:221-225.
49. Casper RC,
Schlemmer RS Jr, Javaid JI. A placebo-controlled crossover study of oral
clonidine in acute anorexia nervosa. Psychiatry Res. 1987;20:249-260.
50. Schvehla TJ,
Mandoki MW, Sumner GS. Clonidine therapy for comorbid attention deficit
hyperactivity disorder and conduct disorder: preliminary findings in a
children's inpatient unit. South Med J. 1994;87:692-695.
51. Ceseña M, Lee DO,
Cebollero AM, Steingard RJ. Case study: behavioral symptoms of pediatric HIV-1
encephalopathy successfully treated with clonidine. J Am Acad Child Adolesc
Psychiatry. 1995;34:302-306.
52. Janicak PG,
Sharma RP, Easton M, et al. A double-blind, placebo-controlled trial of
clonidine in the treatment of acute mania. Psychopharmacol Bulletin.
1989;25:243-245.
53. Kontaxakis V,
Markianos M, Markidis M, Stefanis C. Clonidine in the treatment of mixed
bipolar disorder. Acta Psychiatr Scand. 1989;79:108-110.
54. Bakchine S,
Lacomblez L, Benoit N, et al. Manic-like state after bilateral orbitofrontal
and right temporoparietal injury: efficacy of clonidine. Neurology.
1989;39:777-781.
55. Salín-Pascual R,
de la Fuente J, Fernández-Guardiola A. Effects of clonidine in narcolepsy. J
Clin Psychiatry. 1985;46:528-531.
56. Puzantian T, Hart
LL. Clonidine in panic disorder. Ann Pharmacother. 1993;27:1351-1353.
57. Kinzie JD, Leung
P. Clonidine in Cambodian patients with posttraumatic stress disorder. J Nerv
Ment Dis. 1989;177:546-550.
58. Freedman R, Kirch
D, Bell J, et al. Clonidine treatment of schizophrenia: double-blind
comparison to placebo and neuroleptic drugs. Acta Psychiatr Scand.
1982;65:35-45.
59. Horacek HJ.
Extended-release clonidine for sleep disorders. J Am Acad Child Adolesc
Psychiatry. 1994;33:1210.
60. Rubinstein S,
Silver LB, Licamele WL. Clonidine for stimulant-related sleep problems. J Am
Acad Child Adolesc Psychiatry. 1994;33:281-282.
61. Goldstein S.
Treatment of social phobia with clonidine. Biol Psychiatry. 1987;22:369-372.
62. Schwörer H, Münke
H, Stöckmann F, Ramadori G. Treatment of diarrhea in carcinoid syndrome with
ondansetron, tropisetron, and clonidine. Am J Gastroenterol. 1995;90:645-648.
63. Rovera G,
Furukawa H, Reyes J, et al. The use of clonidine for the treatment of high
intestinal output following small bowel transplantation. Transplantation Proc.
1997;29:1853-1854.
64. Melander M, Almer
S, Ström M. Clonidine in ulcerative colitis and proctitis. J Int Med.
1993;233:93-94.
65. Mikawa K, Nishina
K, Maekawa N, et al. Oral clonidine premedication reduces vomiting in children
after strabismus surgery. Can J Anaesth. 1995;42:977-981.
66. Costello TG,
Cormack JR. Clonidine premedication decreases hemodynamic responses to pin
head-holder application during craniotomy. Anesth Analg. 1998;86:1001-1004.
67. Laurito CE,
Baughman VL, Becker GL, et al. The effectiveness of oral clonidine as a
sedative/anxiolytic and as a drug to blunt the hemodynamic responses to
laryngoscopy. J Clin Anesth. 1991;3:186-193.
68. De Kock M,
Laterre PF, Van Obbergh L, et al. The effects of intraoperative intravenous
clonidine on fluid requirements, hemodynamic variables, and support during
liver transplantation: a prospective, randomized study. Anesth Analg.
1998;86:468-476.
69. Guglielminotti J,
Descraques C, Petitmaire S, et al. Effects of premedication on dose
requirements for propofol: comparison of clonidine and hydroxyzine. Br J
Anaesth. 1998;80:6:733-736.
70. Vallès J, Samsó
E, Vilar X, et al. Pharmacy savings generated by preoperative administration
of clonidine. J Clin Anesth. 1998;10:36-40.
71. Britto JA, McCoy
D, Fourie LR. Clonidine as premedication for rhinoplasty. Plast Reconstr Surg.
1997;100:548-549.
72. Böhrer H, Bach A,
Layer M, Werning P. Clonidine as a sedative adjunct in intensive care.
Intensive Care Med. 1990;16:265-266.
73. Herman VS, Joffe
BI, Kalk WJ, et al. Clinical and biochemical responses to nadolol and
clonidine in hyperthyroidism. J Clin Pharmacol. 1989;29:1117-1120.
74. Moreno Esteban B,
Monereo Mejias S, Rodriguez Poyo-Guerrero P, et al. One year treatment with
clonidine in children with constitutional growth delay. J Endocrinol Invest.
1991;14:75-79.
75. Luke J, Luke DR,
Williams LA, et al. Prevention of cyclosporine-induced nephrotoxicity with
transdermal clonidine. Clin Pharm. 1990;9:49-53.
76. Feder R.
Clonidine Treatment of excessive sweating. J Clin Psychiatry. 1995;56:35.
77. Lucero MA,
McCloskey WW. Alternatives to estrogen for the treatment of hot flashes. Ann
Pharmacother. 1997;31:915-917.
78. Parra RO, Gregory
JG. Treatment of post-orchiectomy hot flashes with transdermal administration
of clonidine. J Urol. 1990;143:753-754.
79. Loprinzi CL,
Goldberg RM, O'Fallon JR, et al. Transdermal clonidine for ameliorating
post-orchiectormy hot flashes. J Urol. 1994;151:634-636.
80. Goldberg RM,
Loprinzi CL, O'Fallon JR, et al. Transdermal clonidine for ameliorating
tamoxifen-induced hot flashes. J Clin Oncol. 1994;12:155-158.
81. Sjoberg RJ,
Simcic KJ, Kidd GS. The clonidine suppression test for pheochromocytoma: a
review of its utility and pitfalls. Arch Intern Med. 1992;152:1193-1197.
82. Camacho-Martinez
F. Localized trichorrhexis nodosa. J Am Acad Dermatol. 1989;20:696-697.
83. Houston MC.
Clonidine hydrochloride: Review of pharmacologic and clinical aspects. Prog
Cardio Dis. 1981;23:337-350.
To comment on this article,
contact editor@uspharmacist.com.






