US Pharm. 2019;44(11)(Specialty&Oncology suppl)):3-10.
ABSTRACT: Hepatitis C is an infection caused by the hepatitis C virus (HCV)that affects the liver and leads to inflammation. Annually, there are approximately 17,000 new chronic HCV cases, and many go undetected until symptoms appear. This virus is the most common blood-borne pathogen in the United States and a leading cause of morbidity and mortality. The landscape of treatment has advanced considerably since the introduction of oral, highly active, direct-acting antivirals in 2013. These improvements in therapy have led to successful eradication of HCV in many cases, when treated early. The goals of treatment focus on eradication of the virus, postponement of fibrosis progression, improvement of symptoms, prevention of complications, reduction in all-cause mortality, and eventually, enhancement in overall patient quality of life. Pharmacists are in a position to educate patients about the etiology, transmission, risk factors, and signs and symptoms of HCV and the various treatment options currently available.
Hepatitis C virus (HCV) is a spherical, enveloped, positive-strand RNA virus that affects the liver and leads to inflammation. First diagnosed in 1989, it has since become a major health issue.1,2 HCV is a major blood-borne human pathogen that can cause both acute and chronic hepatitis, ranging in severity from a mild illness lasting a few weeks to a serious, lifelong condition.3
Typically, new HCV infections are asymptomatic. Some people develop acute hepatitis that does not lead to a life-threatening disease.3 A minority of infected individuals (15% to 45%) spontaneously clear the virus within 6 months of infection without any treatment.3 Most patients who become acutely infected cannot clear the virus and advance to chronic infection; effects include cirrhosis, portal hypertension, hepatic decompensation with encephalopathy, and hepatocellular carcinoma.1 Of those with chronic HCV infection, the risk of cirrhosis ranges from 15% to 30% within 20 years.3,4 Chronic HCV infection frequently follows a progressive course over many years and can ultimately result in the need for a liver transplant.4 Chronic HCV is estimated to affect more than 71 million individuals globally.3,5 In the United States, the CDC estimates that between 2.7 and 3.9 million people (most of whom were born from 1945 through 1965) have chronic HCV.5-8
HCV Genotypes
Because HCV is genetically unstable, it has numerous genotypes and subtypes.9 To date, six major genotypes of HCV have been defined, with more than 50 subtypes.9 The extensive variety of genotypes helps HCV resist the body’s immune system and antiviral medications, making the development of an effective preventive vaccine challenging.9,10 Ongoing research continues to explore a potential vaccine.
In the U.S., the most prevalent HCV genotype is genotype 1, which infects over 80% of U.S. patients who contract HCV, and infects 46% globally.9,11 However, patients can be infected with more than one genotype and, even after treatment, can become reinfected with a different genotype.9 To effectively treat HCV, establishing the genotype is critical, because medications are effective only for specific genotypes.12
As a result of a greater understanding of the pathophysiology of HCV, advances in diagnostic procedures and healthcare interventions, general sanitary improvements (testing of donated blood, training of health personnel), and the introduction of new antiviral drugs in the last few years, many patients with HCV, if treated properly, can now be cured.13-15
The introduction in 2013 of oral, direct-acting antivirals (DAAs) that target multiple mechanisms of the HCV life cycle has led to great optimism that HCV elimination is attainable.13,16,17 DAAs have a cure rate of more than 95%, a treatment duration of only 8 to 12 weeks, and fewer and less severe adverse effects than their predecessors.3,16 As a result of advances in therapy, reports indicate that in 2019, HCV is no longer the primary indication for liver transplantation.13 Moreover, DAAs are also being prescribed to treat transplant recipients without HCV who get organs from donors with HCV infection, augmenting organ availability for transplant.13 The World Health Organization (WHO) has called for the elimination of viral hepatitis as a major public health threat by 2030.13
Signs and Symptoms of HCV
In the majority of those with HCV, the virus results in varying levels of hepatic inflammation and fibrosis.9 Additionally, HCV infection induces immunoregulatory and proinflammatory pathways that may add to liver fibrosis.8,9 The incubation period for HCV ranges from 2 weeks to 6 months.3,9 Although most patients with HCV infection are asymptomatic, 20% to 30% of those newly infected experience fatigue, abdominal pain, anorexia, or jaundice.6,9 In many patients, extrahepatic indicators of HCV involve the joints, muscles, and skin, and are among the initial signs and symptoms.9 In advanced or decompensated hepatic disease, which is related to hepatic dysfunction and portal hypertension, signs and symptoms include pruritus, jaundice, hepatic encephalopathy, edema, ascites, and hematemesis or melena.8,9,17
Modes of Transmission
The most prevalent mechanism of transmission of HCV is the percutaneous route through IV drug use with nonsterile needles; the transfusion of unscreened blood and blood products; or as a consequence of poor compliance with universal precautions in the healthcare setting.6,18 Transfusion of blood contaminated with HCV was once a chief cause of HCV transmission. However, since 1992, the screening of donated blood for HCV antibody has dramatically diminished the risk of transfusion-associated HCV infection.5
In developed countries, the majority of new HCV infections are reported in injection-drug users (IDUs).5 The most recent surveys of active IDUs in the U.S. suggest that:
• About 33% of young IDUs (aged 18-30 years) are HCV-infected5,6,18
• Older and former IDUs usually have a far larger incidence (approximately 70%-90%) of HCV infection, owing to a lower level of awareness of the risks of blood-borne viruses that existed prior to implementation of public-education programs5
• In some cases, a risk factor for HCV infection cannot be uncovered; these cases are probably related to percutaneous inoculations that are not evident.18,19
Diagnosis of Hepatitis C
The diagnosis of chronic HCV is suspected when:3,19,20
• A patient exhibits symptoms and signs
• Increases in aminotransferase levels are incidentally observed
• There is a previous diagnosis of acute hepatitis.
Diagnostic tests for HCV can be separated into two general categories: serologic assays, which detect antibodies to HCV, and molecular assays, which detect HCV RNA.9 HCV antibodies can be noticed in the blood 2 to 3 weeks after exposure.9,10 If an antibody test is negative, HCV infection is doubtful in most patients and additional testing may not be warranted. Nevertheless, HCV antibodies may not be measurable in some patients with HCV infection; for example, those who are severely immunocompromised and those on hemodialysis.9 HCV RNA testing is warranted for these types of patients.9,10
After HCV infection has been established, providers should continue with further testing to determine the genotype and extent of hepatic damage; the degree of hepatic damage helps guide treatment.12,21 For example, some pharmacologic agents are not recommended for advanced disease, such as decompensated cirrhosis, or if the patient is pregnant or lactating.9,12
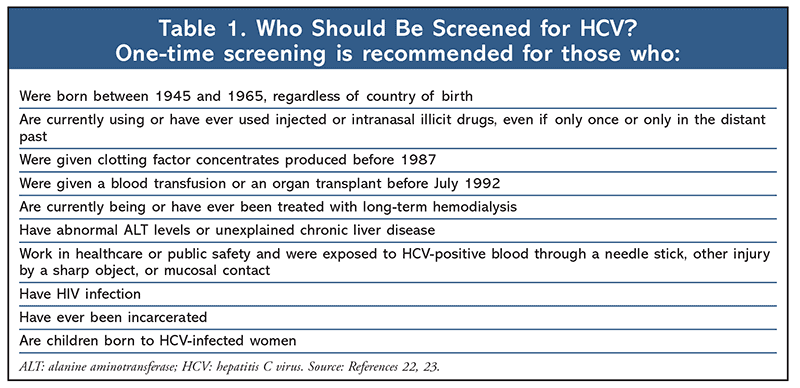
Individuals with certain characteristics should be screened for HCV, whether they have suggestive symptoms or not. Such testing is critical because symptoms may not develop until HCV has significantly damaged the liver, years after the initial infection. For a guide to who should be screened for HCV, see TABLE 1.22,23
Treatments for Hepatitis C
The DAAs offer improved efficacy when combined with both pegylated interferon (PEG-IFN) and ribavirin, particularly for genotype 1 infections.5,6 The first protease inhibitors, boceprevir and telaprevir, have been discontinued and replaced by antivirals with better efficacy and enhanced safety profiles.5,6 The goals of treatment are to eradicate virus, delay fibrosis progression, alleviate symptoms, prevent complications, limit all-cause mortality, and eventually improve patient quality of life.1,9,24
The guidelines for the current diagnostic workup and management of HCV continue to evolve rapidly.5 Clinicians are advised to refer frequently to the most recent recommendations of the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA).12
According to the CDC, there is no recommended treatment for acute HCV. Individuals with acute HCV infection should be followed by a doctor and only considered for treatment if their infection remains and becomes chronic.6
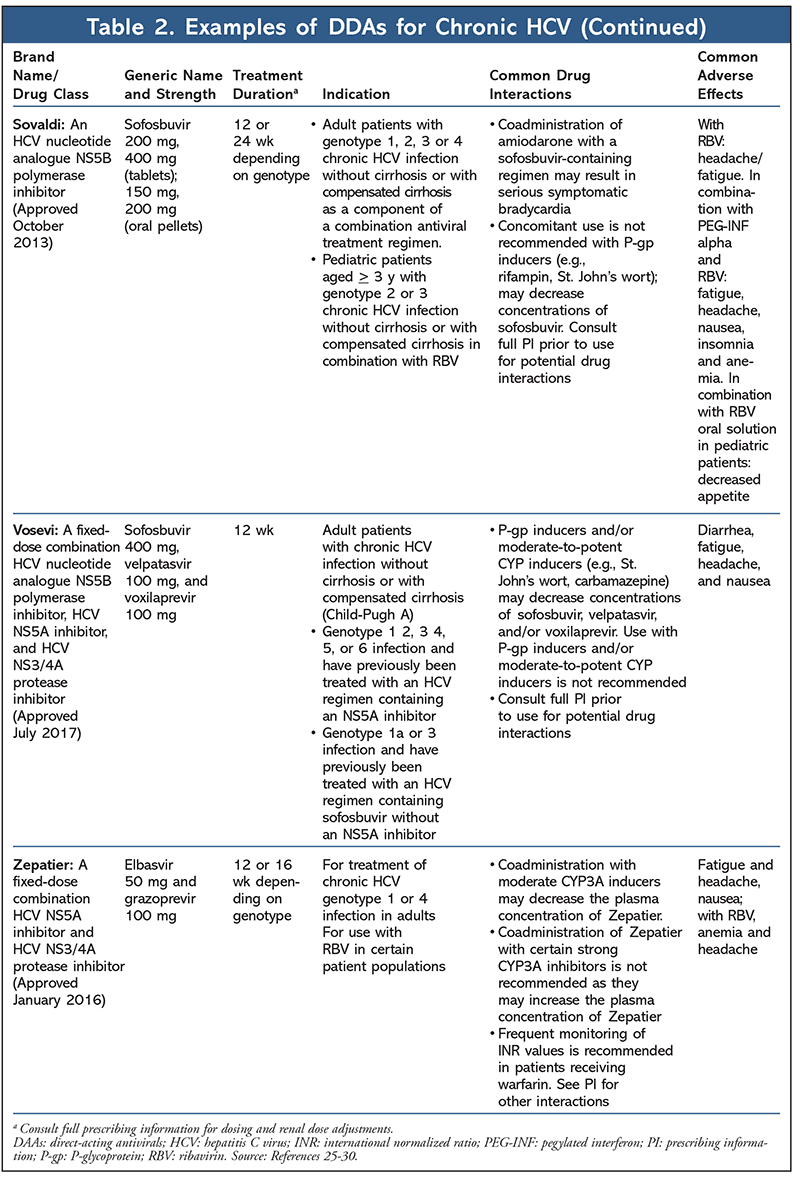
There are several classes of approved DAAs: NS3/4A protease inhibitors, NS5A inhibitors, and polymerase inhibitors (nonnucleoside inhibitors and nucleotide inhibitors).5,6 The specific combination of antivirals prescribed is determined by genotype (TABLE 2).25-30 Compared with other genotypes, genotype 1 is more resistant to conventional treatment with dual therapy with PEG-IFN-alpha plus ribavirin.5,20 However, due to the use of IFN-free regimens of oral DAAs, the percentage of sustained virologic response (SVR) has expanded from less than 50% to up to 95%.20 First-line regimens include novel fixed-dose combinations5,20:
• Ledipasvir/sofosbuvir
• Elbasvir/grazoprevir
• Velpatasvir/sofosbuvir
• Glecaprevir/pibrentasvir.
Because of the success rate for eradication of HCV and the favorable safety profile associated with DAAs, current guidelines recommend treatment for all individuals infected with HCV unless life expectancy is less than 1 year due to non–HCV-related comorbidities.12,13 Recommended HCV treatment is for a limited duration (8-12 weeks).13 If HCV RNA is under the measurable limit 12 weeks after completion of DAA therapy, SVR has been achieved and HCV infection is considered cured.13 If measurable HCV RNA returns within 12 weeks of treatment cessation, it is considered a relapse, and treatment-emergent resistance to some or all of the drugs in the unsuccessful treatment regimen is possible.13 While relapse after SVR at 12 weeks is rare, guidelines recommend confirming SVR 24 to 48 weeks after therapy.12,13 More frequently, viremia after SVR at 12 weeks represents a reinfection (new exposure and HCV acquisition).13,31
Recent Hepatitis C Developments
In August 2019, the FDA issued a cautionary message indicating that HCV drugs Mavyret, Zepatier, and Vosevi can, in rare cases, actually lead to the worsening of hepatic function or to hepatic failure when used to treat HCV in patients with moderate-to-severe liver disease, for which the drugs are not indicated.32
Also in August, the United States Preventive Services Task Force announced a draft recommendation for screening all adults aged 18 to 79 years for HCV, updating their more limited 2013 screening recommendation and citing the prevalence of HCV among younger adults and the existence of better treatment with the DAAs.33
Prevention of HCV
Currently, there is no effective vaccine against HCV; therefore the WHO has recommended the following measures for primary prevention3:
• Safe and appropriate use of healthcare injections
• Safe handling and disposal of sharps and waste
• Provision of comprehensive harm-reduction services to individuals who inject drugs including sterile injecting equipment and effective treatment of dependence
• Testing of donated blood for hepatitis B virus and HCV (as well as HIV and syphilis)
• Training of health personnel
• Hand hygiene, including surgical hand preparation, hand washing, and use of gloves
For individuals infected with HCV, the WHO recommends3:
• Education and counseling on options for care and treatment
• Immunization with the hepatitis A and B vaccines to prevent coinfection from these hepatitis viruses and to protect the liver
• Early and appropriate medical management including antiviral therapy; and
• Regular monitoring for early diagnosis of chronic liver disease.
The Role of the Pharmacist
Because there is a significant potential for drug-drug interactions that may impact the effectiveness of HCV medications, pharmacists are in a pivotal position to identify these interactions and make clinical interventions accordingly. Pharmacists should conduct a thorough patient profile review and make sure that the patient provides a comprehensive list of all their current medications including prescription, nonprescription, and complementary and alternative medications, to ascertain if there are any interactions that would affect the efficacy of the HCV regimen or result in toxicity. This critical task is essential to averting potential drug-drug interactions or contraindications. For example, the absorption of some DAAs (e.g., ledipasvir/sofosbuvir and sofosbuvir/velpatasvir) is diminished by some OTC agents such as proton pump inhibitors, histamine2 receptor antagonists, and antacids; many patients may not think to disclose this information to their healthcare providers.34 As another example, some statins must be dose-restricted when used in combination (e.g., glecaprevir/pibrentasvir and elbasvir/grazoprevir) owing to a potential increase in concentration of the statin that may augment the risk of myopathy.34 An excellent and comprehensive drug-interactions resource to explore HCV drug-drug interactions can be found in Reference 35.34,35
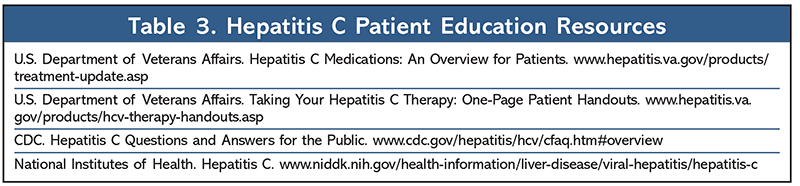
As front-line healthcare providers, pharmacists can act as patient identifier, patient educator (see TABLE 3 for resources), and patient advocate. During counseling, pharmacists can improve clinical outcomes by providing patients with pertinent information about their drug therapy, including the critical nature of patient adherence. Patients should be informed of the proper administration of prescribed drug therapy and any possible adverse effects or drug interactions. To avoid possible drug interactions, patients should talk with their pharmacist or primary healthcare provider before taking any other medications, including nonprescription medications and nutritional supplements. The emergence of the fixed-dose combination oral DDAs has revolutionized the treatment landscape for HCV and pharmacists can encourage patients to get tested and get treated immediately if they suspect they have HCV.
REFERENCES
1. Basit H. Hepatitis C. StatPearls. National Center for Biotechnology. Published May 15, 2019. www.ncbi.nlm.nih.gov/books/NBK430897. Accessed August 28, 2019.
2. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
3. World Health Organization. Hepatitis C. Published July 9, 2019. www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed August 28, 2019.
4. Bhamidimarri KR, Satapathy SK, Martin P. Hepatitis C virus and liver transplantation. Gastroenterol Hepatol (NY). 2017;13(4):214-220.
5. Dhawan VK. Hepatitis C. Practice essentials, background, pathophysiology. Medscape. Published May 30, 2019. https://emedicine.medscape.com/article/177792-overview. Accessed August 28, 2019.
6. CDC. Viral hepatitis. Q&As for health professionals. Reviewed July 2, 2019. www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed August 28, 2019.
7. Dhawan VK. What is the prevalence of hepatitis C (hep C) infection? Latest medical news, clinical trials, guidelines. Medscape. Published May 30, 2019. www.medscape.com/answers/177792-3820/3820-answer. Accessed August 28, 2019.
8. Kim AY. Epidemiology, natural history, and diagnosis of hepatitis C in the HIV-infected patient. UpToDate. 2017. www.uptodate.com/contents/epidemiology-natural-history-and-diagnosis-of-hepatitis-c-in-the-hiv-infected-patient. Accessed August 28, 2019.
9. Chaney A. Caring for patients with chronic hepatitis C infection. Nursing 2019. 2019; 49(3):36-42.
10. Chopra S. Characteristics of the hepatitis C virus. UpToDate. 2019. www.uptodate.com/contents/characteristics-of-the-hepatitis-c-virus.
11. Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014; 61(1suppl):S45-S57.
12. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV guidelines. HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.HCVguidelines.org. Accessed October 10, 2019.
13. Marks K, Naggie S. Management of Hepatitis C in 2019. JAMA. May 17. 2019. Epub ahead of print.
14. Rosenberg ES, Rosenthal EM, Hall EW, et al. Prevalence of hepatitis C virus infection in US states and the District of Columbia, 2013 to 2016. JAMA Netw Open. 2018;1(8):e186371.
15. Moyer VA; US Preventive Services Task Force. Screening for hepatitis C virus infection in adults. Ann Intern Med. 2013;159(5):349-357.
16. Douglass CH, Pedrana A, Lazarus JV, et al. Pathways to ensure universal and affordable access to hepatitis C treatment. BMC Med. 2018;16:175.
17. Doyle JS, Thompson AJ, Higgs P, et al. New hepatitis C antiviral treatments eliminate the virus. Lancet. 2017;390:358-359.
18. Puchades Renau L, Berenguer M. Introduction to hepatitis C virus infection: overview and history of hepatitis C virus therapies. Hemodial Int. 2018;22(suppl 1):S8-S21.
19. Li HC, Lo SY. Hepatitis C virus: virology, diagnosis and treatment. World J Hepatol. 2015;7(10):1377-1389.
20. Rutherford AE. Hepatitis C, chronic. Merck Manuals Professional Edition. Reviewed January 2019. www.merckmanuals.com/professional/hepatic-and-biliary-disorders/hepatitis/hepatitis-c,-chronic. Accessed October 3, 2019.
21. Conde I, Vinaixa C, Berenguer M. Hepatitis C-related cirrhosis. Current status. Med Clin (Barc). 2017;148(2):78-85.
22. CDC. Testing recommendations for hepatitis C virus infection. www.cdc.gov/hepatitis/hcv/guidelinesc.htm. 2015. Accessed October 2, 2019.
23. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV testing and linkage to care. Updated May 24, 2018. www.hcvguidelines.org/evaluate/testing-and-linkage. Accessed October 2, 2019.
24. Feld JJ. Extending a helping hand: addressing hepatitis c in economic migrants and refugees. Ann Hepatol. 2018;17(1):8-10.
25. Epclusa (sofosbuvir) package insert. Foster City, CA: Gilead Science, Inc; 2017.
26. Harvoni (ledipasvir/sofosbuvir)) package insert. Foster City, CA: Gilead Science, Inc; 2019.
27. Mavyret (glecaprevir/pibrentasvir) package insert. North Chicago, IL: AbbVie; 2019.
28. Sovaldi (sofosbuvir) package insert. Foster City, CA: Gilead Science, Inc; 2019
29. Vosevi sofosbuvir/velpatasvir/voxilaprevir) package insert. Foster City, CA: Gilead Science, Inc; 2019.
30. Zepatier (elbasvir/grazoprevir) package insert. Whitehouse Station, NJ: Merck Sharpe & Dohme; 2018.
31. Sarrazin C, Isakov V, Svarovskaia ES, et al. Late relapse versus hepatitis C virus reinfection in patients with sustained virologic response after sofosbuvir-based therapies. Clin Infect Dis. 2017;64(1):44-52.
32. FDA. Center for Drug Evaluation and Research. FDA warns about rare occurrence of serious liver injury with use of hepatitis C medicines Mavyret, Zepatier, and Vosevi in some patients with advanced liver disease. Published August 28, 2019. www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-occurrence-serious-liver-injury-use-hepatitis-c-medicines-mavyret-zepatier-and. Accessed August 28, 2019.
33. Walker M. USPSTF: Screen all adults for hepatitis C infection. Med Page Today. Published August 27, 2019. www.medpagetoday.com/infectiousdisease/hepatitis/81828. Accessed August 28, 2019.
34. Spooner L. Pharmacists’ role in HCV care: incorporating medication therapy management. Fundamentals of pharmacy-based HIV and HCV prevention and care. Published August 27, 2019. www.clinicaloptions.com/hepatitis/programs/pharmacy-care/clinicalthought/ct6/page-1. Accessed October 3, 2019.
35. Sullivan KM, Spooner LM, Harris E, et al. A bitter pill to swallow: why medication safety is critical in hepatitis C treatment. P T. 2018;43(12):764-768.
To comment on this article, contact rdavidson@uspharmacist.com.