US Pharm. 2021;46(4):17-20.
ABSTRACT: IV antibiotics are generally thought to be the mainstay of treatment for bloodstream infections due to their high bioavailability and fast peak plasma levels. However, the ideal route of administration of any medication is one that achieves serum concentrations sufficient to produce the desired result without any unwanted effects. Associated costs and risks with IV agents make them less than ideal in many infectious diseases, including bacteremia. Oral therapy offers several benefits with similar outcomes, and the decision to treat a patient with oral antibiotics should be based on current evidence as well as patient, pathogen, and drug characteristics.
Bacterial bloodstream infections (BSIs) are a considerable cause of morbidity and mortality, with nearly 30 million cases worldwide each year resulting in 6 million deaths and insurmountable costs.1 IV antibiotics are generally thought to be the mainstay of treatment for BSIs with their high bioavailability and fast peak plasma levels. This mantra is indeed warranted in acutely ill patients, such as those with sepsis, as timely IV antibiotics can be lifesaving.2
However, the clinically stable patient may benefit from partial or complete oral antibiotics by improving their mobility, reducing the risk of IV catheter–related infections and discomfort, as well as reducing healthcare costs. Prolonged catheter placement can cause numerous complications, including infection, thrombosis, and device dysfunction.3,4 The at-home utilization of these devices also increases the risks of complications associated with patient errors in their appropriate care and management.
In 2012, the American Board of Internal Medicine Foundation launched the “Choosing Wisely” campaign aimed to promote practice changes to improve patient health and safety by avoiding unnecessary diagnosis and treatments.5 The organization recommends that providers “prefer oral formulations of highly bioavailable antimicrobials whenever possible.” This and several other initiatives by organizations such as the CDC and World Health Organization have launched the implementation of antimicrobial stewardship (AMS) programs and research to prevent further overuse of antimicrobials and antibiotic resistance. Essential AMS principles set forth by the CDC include the use of the shortest length of antibiotic treatment and decreasing utilization of broad-spectrum antimicrobials.6,7
Oral antimicrobial therapy for BSI, when appropriate, not only meets the recommendations of these organizations, but it also provides reductions in patient and healthcare costs and complications while providing noninferior outcomes. However, the practicality of oral therapy must be assessed carefully with each patient based on the organism, pharmacokinetics, patient factors (such as drug allergies and ability to take oral medications), clinical stability, and available evidence.
Assessing the Appropriateness of IV to Oral Conversion of Antibiotics
For many medications, the bioavailability of the oral formation is comparable if not equal to that of their IV counterpart.4 However, there are several factors to consider and follow when approaching the conversion of a patient from IV to oral therapy while treating BSI. TABLE 1 illustrates the three main types.8
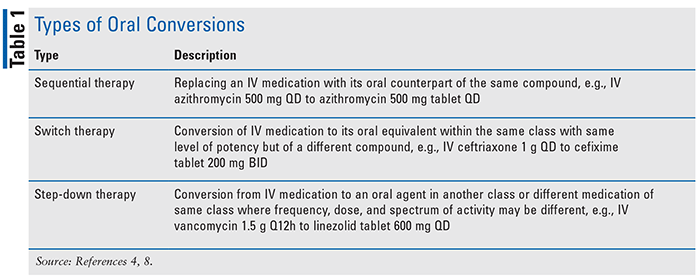
Source Control: For all patients undergoing treatment for infection, adequate source control is paramount.9 Without the appropriate physical measures to remove, debride, or amputate the affected area(s), the use of any antibiotics (IV or oral) is inconsequential. Before recommending an oral conversion, it is important to assess the level of source control achieved and ensure repeat blood cultures continue to be negative. As with any therapy change, appropriate follow-up measures should be implemented to monitor a patient’s clinical status (white blood cell count, culture reports, vitals, etc.).
Sensitivities: Many bacteria are intrinsically susceptible to antibiotics, but there is always potential for multidrug resistance.10 Therefore, it is crucial to refer to sensitivity reports to ensure the pathogen is susceptible to an oral antibiotic before transitioning. For example, many Gram-negative species are intrinsically susceptible to beta-lactams, but with the increasing emergence of extended-spectrum beta-lactamase enzymes, antimicrobial therapy is limited and oral medications may be inappropriate.11
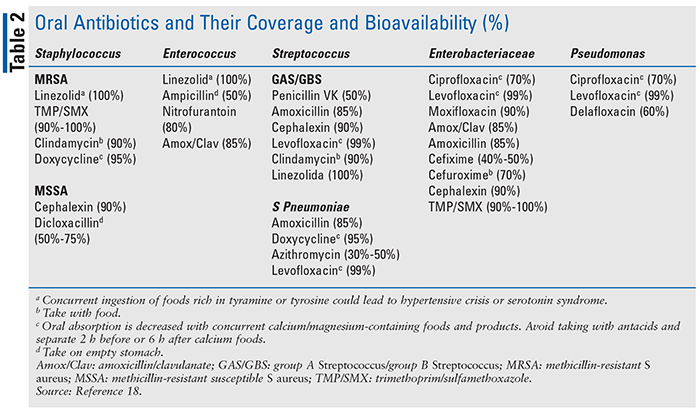
Pharmacokinetics: A retrospective cohort study found that oral antibiotics with high bioavailability (≥95%) were effective treatments in hospitalized patients with Gram-negative bacteremia. The ideal oral antibiotic should have excellent oral bioavailability in order to achieve adequate blood concentrations. Agents that do not have 100% bioavailability require higher doses to treat bacteremia than other types of infections. TABLE 2 lists oral antibiotics and their coverage of common bacteria involved in BSI. The oral absorption of several antibiotics are affected by certain foods and medication administration, so it is important to consider these interactions to achieve maximum concentrations.
Gram-Positive Bloodstream Infections
Methicillin-Resistant Staphylococcus Aureus: Staphylococcus aureus, including methicillin-resistant and susceptible S aureus (MRSA and MSSA, respectively), is a leading cause of bacteremia in North America and the most common pathogen of Gram-positive bacteremia. Over 30% of the population is colonized with S aureus.12 In the 2011 Infectious Diseases Society of America (IDSA) guidelines, first-line antimicrobial therapy for uncomplicated MRSA BSI (defined as those without infective endocarditis, negative follow-up cultures within 2 to 4 days, and no evidence of metastatic sites of infection) include IV vancomycin or daptomycin for at least 2 weeks.13 Complicated cases are recommended to have 4 to 6 weeks of therapy with the same agents. While the guidelines do not include recommendations on oral therapy for MRSA bacteremia, there have been recent studies highlighting the positive potential for transitioning uncomplicated patients from IV to oral antibiotics.
In a single-center, retrospective, observational cohort study by Jorgensen and colleagues, 492 patients with MRSA BSI were included and separated into two comparator arms of oral versus IV antibiotics in the outpatient setting.14 The primary outcome was 90-day clinical failure (MRSA BSI recurrence, deep-seated MRSA infection, or all-cause mortality). Before patients were discharged from the hospital to complete their respective outpatient therapies, MRSA BSI clearance was mandatory. The most common oral therapy was linezolid (50%), followed by trimethoprim/sulfamethoxazole (34%) and clindamycin (15.7%). The difference in 90-day failure was not significant in the oral-therapy group compared with the IV group (hazard ratio [HR] 0.379, 95% CI, 0.131-1.101) and patients in the oral group had a reduced rate of 90-day hospital readmission (HR 0.603, 95% CI, 0.388-0.937).
Willekens et al compared an early switch to linezolid between Days 3 and 9 of treatment to full standard parenteral therapy in a prospective study of 135 patients with uncomplicated MRSA BSI.15 There was no difference found in 90-day relapse between the two groups nor in 30-day all-cause mortality. In addition, the median length of hospital stay was significantly shorter in the linezolid group versus the standard parental therapy group (8 vs. 19 days, P <.01).
Evidence found in these studies support the safety and efficacy of transitioning a patient with uncomplicated MRSA BSI from IV to oral therapy for the remaining duration of treatment in order to decrease hospital length of stay and healthcare costs while achieving the same outcome. To emphasize, it is important that only uncomplicated infections be considered for oral antibiotics, and infective endocarditis must be ruled out when treating Gram-positive BSI.14 Oral linezolid and trimethoprim/sulfamethoxazole have favorable pharmacokinetics with 100% bioavailability, and a 2005 pooled analysis demonstrated noninferiority of linezolid to vancomycin for S aureus BSI.16 Other agents with activity against MRSA, such as clindamycin and doxycycline, have limited data in treating BSI, and the 2011 IDSA guidelines advise against their use in this setting.14
Enterococcus Species: Another important cause of Gram-positive BSI is Enterococcus species, specifically E faecalis and E faecium.17 Currently, there is a lack of evidence to support oral antibiotics for bacteremia caused by these organisms. Although the preferred IV treatment is ampicillin for susceptible strains, oral ampicillin has poor bioavailability (50%) and has not been studied.18 Several other oral antibiotics, including amoxicillin, amoxicillin/clavulanate, and nitrofurantoin (TABLE 2) have activity against Enterococcus species, but these also do not achieve high enough concentrations to be effective against BSI.18 While IV ampicillin remains the drug of choice for susceptible Enterococcus infections, few options remain to treat systemic multidrug-resistant infections (e.g., vancomycin-resistant Enterococcus [VRE]), which include linezolid, daptomycin, and tigecycline.19 In a meta-analysis of 10 retrospective studies comparing IV linezolid and daptomycin in the treatment of VRE BSI, patients treated with daptomycin had significantly higher 30-day all-cause mortality and infection-related mortality.20 More research is needed to support oral linezolid in this setting, but it is reasonable to suggest that with its 100% bioavailability that it is an effective oral treatment option in the uncomplicated patient.
Streptococcus Species: This final group of Gram-positive bacteria commonly found in BSI includes S pneumonia and Group A (S pyogenes) and Group B (S agalactiae) Streptococcus. Fluoroquinolones are favorable oral treatments for Streptococcus species with high bioavailability, but they are not without inherent safety concerns such as tendonitis and aortic dissection. Beta-lactams are another fair choice as Streptococcus is generally susceptible to these antibiotics. Beta-lactams do have poor bioavailability (50%-85%), but a multicenter retrospective study found that oral step-down therapy with a beta-lactam was noninferior to fluoroquinolones in the treatment of uncomplicated streptococcal BSI.21 Linezolid has bactericidal activity against Streptococcus and is another appreciative oral agent in bacteremic patients.18
Gram-Negative Bloodstream Infections
Enterobacteriaceae: There are over 100 species of Enterobacteriaceae, but the most common pathogens in this family to cause BSI include Escherichia coli, Enterobacter sp., Klebsiella sp., Proteus mirabilis, and Serratia marcescens.22 Gram-negative bacteria are not likely to cause complicated sequelae, such as endocarditis, compared with Gram-positive organisms, due to being less adherent to cardiac valve surfaces.23 Traditionally, the duration of treatment for BSIs with Gram-negative organisms has ranged from 7 to 14 days, and newer evidence suggests that 7 days is optimal, with several studies observing no differences in outcomes with shorter therapies.24 The optimal route of antibiotic administration has not been established; however, several recent studies have indicated oral therapy is noninferior to IV.
Rieger and colleagues retrospectively examined 241 patients who were either treated exclusively with IV antibiotics compared with partial or complete oral therapy for Enterobacteriaceae bacteremic UTIs.25 The results found no difference in treatment failure, and those treated with oral antibiotics had a shorter median length of hospital stay. This study, along with most current data supporting conversion to oral antibiotics for Gram-negative BSI, are limited to those secondary to urinary tract sources.25 Most evidence supporting oral antibiotics for Gram-negative bacteremia are from urinary sources.
In a more recent retrospective cohort study by Tamma et al, 30-day mortality was assessed in 1,478 hospitalized patients with monomicrobial Enterobacteriaceae BSI due to a variety of infectious sources, including pulmonary, intraabdominal, and catheter-associated.26 Patients were either treated with complete IV therapy or converted to oral therapy within 5 days of their IV treatment following source control. The primary outcome of 30-day mortality was not significantly different between the two groups. There was also no difference in 30-day recurrence of bacteremia, and patients in the oral therapy group were discharged from the hospital an average 2 days sooner.
After assessing the appropriateness of treating a bacteremic patient due to Enterobacteriaceae with oral antibiotics, the ideal antimicrobial would be those with high bioavailability. A retrospective cohort study by Kutob et al found that oral antibiotics with high bioavailability (³95%) were effective step-down treatments in hospitalized patients with Gram-negative bacteremia, and these agents are reasonable alternatives in clinical practice provided sufficient susceptibility.27
Pseudomonas aeruginosa: P aeruginosa is an opportunistic Gram-negative pathogen responsible for a large majority of nosocomial infections. Oral agents used to treat P aeruginosa are limited to fluoroquinolones (TABLE 2), but this bacterial species can display multiple mechanisms of resistance, and inappropriate treatment of BSI is associated with high hospital mortality.28,29 There are no data to support oral antibiotics in the treatment of P aeruginosa bacteremia, and the use of IV therapy should be used for the entire duration of treatment.
The Pharmacist’s Role
As the role of the pharmacy profession is rapidly expanding, pharmacists are placed in an optimal position to assess when oral antibiotics are appropriate in treating the patient with bacteremia. With the knowledge of pharmacokinetics and antibiotic mechanisms of action, pharmacists can make evidence-based decisions and recommendations about drug therapy as well as following up with providers for patient monitoring and clinical status. It is also important to recognize the source of infection and the evidence associated with treating bacteremia from these different sources. Pharmacists in both the inpatient and community setting, such as home infusion centers, can assist providers with the transition to oral antibiotics.
Not every patient with a bacterial BSI can be treated with oral antibiotics. The appropriateness of this route of administration depends on the organism, source control, patient factors, and pharmacokinetics. However, many BSIs can be effectively treated with partial or complete oral therapy, saving in healthcare and patient costs, avoiding complications, and promoting antimicrobial stewardship.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Fleischmann C, Scherag A, Adhikari NKJ, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Amer J Resp and Crit Care Med. 2016(193);3:259–272. 2. Youkee D, Hulme W, Roberts T, et al. Time matters: antibiotic timing in sepsis and septic shock. Crit Care Med. 2016;44(10):e1016-e1017.
3. Patel AR, Patel AR, Singh S, et al. Central line catheters and associated complications: a review. Cureus. 2019;11(5):e4717.
4. Cyriac JM, James E. Switch over from intravenous to oral therapy: a concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87.
5. Lehmann C, Berner R, Bogner JR, et al. The “choosing wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268.
6. The World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015.
7. Centers for Disease Control and Prevention. Antibiotic Use in the United States, Progress and Opportunities, 2018.
8. Kuper KM. Intravenous to oral therapy conversion. Textbook of Competence Assessment Tools for Health-System Pharmacies. American Society of Health-System Pharmacists. 4th ed. 2008:347-360.
9. Marshall JC, al Naqbi A. Principles of source control in the management of sepsis. Crit Care Clin. 2009;25(4):753-68.
10. Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4(3):482-501.
11. Brook I. Beta-lactamase producing bacteria and their role in infection. Rev in Med Microb. 2005;16(2):91-99.
12. Tong SY, Davis JS, Eichenberger E, et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603-661.
13. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3): e18-e55.
14. Jorgensen SCJ, Lagnf AM, Bhatia S, et al. Sequential intravenous-to-oral outpatient antibiotic therapy for MRSA bacteraemia: one step closer. J Antimicrob Chemother. 2019;74(2):489-498.
15. Willekens R, Puig-Asensio M, Ruiz-Camps I, et al. Early oral switch to linezolid for low-risk patients with Staphylococcus aureus bloodstream infections: a propensity-matched cohort study. Clin Infect Dis. 2019;69(3):381-387.
16. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929.
17. Giannella M, Bartoletti M, Gatti M, Viale P. Advances in the therapy of bacterial bloodstream infections. Clin Microbiol Infect. 2020 Feb;26(2):158-167. Epub 2019 Nov 14.
18. Gilbert DN, Chambers HF, Eliopoulos GM, et al. Sanford Guide To Antimicrobial Therapy. 50th ed. Dallas, TX: Antimicrobial Therapy, Inc. 2020.
19. Gao W, Howden BP, Stinear TP. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr Opin Microbiol. 2018;41:76-82.
20. Balli EP, Venetis CA, Miyakis S. Systematic review and meta-analysis of linezolid versus daptomycin for treatment of vancomycin-resistant Enterococcal bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739.
21. Arensman K, Shields M, Beganovic M, et al. Fluoroquinolone versus beta-lactam oral step-down therapy for uncomplicated Streptococcal bloodstream infections. Antimicrob Agents Chemother. 2020;64(11):e01515- e01520.
22. Al-Hasan MN, Eckel-Passow JE, Baddour LM. Impact of healthcare-associated acquisition on community-onset gram-negative bloodstream infection: a population-based study: healthcare-associated gram-negative BSI. Eur J Clin Microbiol Infect Dis. 2012;31(6):1163-1171.
23. Baddour LM, Wilson WR, Bayer AS. Infective endocarditis in adults: diagnosis, antimocrobial therapy, and management of complications. Circulation. 2015;132:1435-1486.
.24. Yahav D, Franceshini E, Koppel F, et al. Bacteremia duration study group. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: a non-inferiority randomized controlled trial. Clin Infect Dis. 2019;69(7):1091-1098.
25. Rieger KL, Bosso JA, MacVane SH, et al. Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy. 2017;37(11):1479-1483.
26. Tamma PD, Conley AT, Cosgrove SE, et al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019;179(3):316-323.
27. Kutob LF, Justo JA, Bookstaver PB, et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503.
28. Pang Z, Raudonis E, Glick BR, et al. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Botechnol Adv. 2019;37(1):177-192.
29. Micek ST, Lloyd AE, Ritchie DJ, et al. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311.
To comment on this article, contact rdavidson@uspharmacist.com.






