US Pharm.
2006;11:70-76.
The use of complementary and
alternative medicine (CAM) has drastically increased over the past few years.
In 2002, it was estimated that 62% of all Americans use some form of CAM.1
CAM spending rose 12% to $30 billion in 2001 and accounted for almost 2.5% of
the $1.2 trillion spent in personal health care in the United States.2
People are seeking other health options and are coming to pharmacists to find
answers to their many health questions. One CAM area of interest is the use of
probiotic agents. Consumers use probiotic agents for a variety of disease
states, including antibiotic-associated diarrhea, irritable bowel syndrome,
and atopic eczema. In the U.S., probiotic sales rose to $177 million in 2003,
were estimated at $764 million in 2005, and are expected to rise at an average
annual growth rate of 7.1%, to reach $1.1 billion in 2010.3 This
article focuses on the non–FDA-approved use of probiotics in the treatment of
atopic eczema in children. In addition, this article discusses the recommended
dosage and possible efficacy of probiotic strains.
The idea of using probiotics
is not a new concept. In the early 1900s, Russian scientist Elie Metchnikoff
hypothesized that by increasing fermented milk intake, a person might increase
his or her life span and health. Metchnikoff observed Bulgarian peasants who
lived long, healthy lives and noted their consumption of fermented milk
products. He was convinced that yogurt contained the bacteria necessary to
protect intestines from the damaging impact of pathogens.4 This
hypothesis is based on the effects of the bacteria used to ferment milk, not
the milk itself. In 1908, Metchnikoff won the Nobel Prize for his work in
immunology. His theory that certain bacteria may improve health is still of
major interest today.
Numerous types of bacteria and
yeasts have been studied for their probiotic potential. A probiotic is
defined as viable bacteria that colonizes the intestines and modifies the
intestinal microflora and their metabolic activities. Probiotics are often
referred to as the "friendly bacteria." The name probiotics
literally means "for life." There are preparations of products containing
living, specified microorganisms in adequate numbers. They help restore the
indigenous microflora and exert a health benefit to the host. Probiotics are
found naturally in our intestinal tract and are thought to help stabilize the
gut microflora. These microbes are often delivered as a lyophilized powder in
a capsule or suspended in yogurt or a dairy beverage.5
Origin of Intestinal Flora
The human
intestinal tract is sterile at birth.6 After birth, infants are
exposed to microorganisms, and over time, hundreds of bacteria reside within
the intestinal tract. Exposure to these different types of flora is thought to
depend on the type of delivery (vaginal or surgical), source of nutrition
(bottle or breast), gestational age, other human contact, environment, and the
mother's prenatal dietary intake.7
Regarding type of birth, it is
proposed that infants born vaginally are exposed to intestinal flora via the
birth canal. These infants appear to have a head start on colonization of
intestinal flora. Babies born by cesarean do not pass through the birth canal
and are believed to have delayed colonization of these organisms.6
Studies have shown that breast-fed infants have higher concentrations of
microflora in the first weeks of life, whereas formula-fed infants have much
fewer of these organisms.6
Once intestinal flora is
established, it is relatively stable throughout life. Some temporary
alterations include the induction of antibiotic therapy, radiation therapy,
and chemotherapy.6
The Role of Microflora
The human body
relies on normal microflora for many functions, including metabolizing foods
and certain drugs, absorbing nutrients, and preventing colonization of
pathogenic bacteria.7 The adult body contains 1 x 1014 cells,
of which 90% are accounted for by members of the microflora.5 These
microflora are considered the first line of defense against invasion by
pathogenic organisms. Sufficient colonization of microflora has been shown to
be an extremely effective natural barrier against pathogens such as Clostridium
difficile, Salmonella, Shigella, Pseudomonas, Escherichia coli, and
Candida albicans. Probiotics are the most effective against conditions
that are linked to the underlying disruption of this protective normal
microflora barrier (e.g., due to broad-spectrum antibiotics, travel, stress,
and changes in nutrition).5
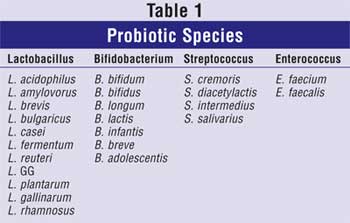
Probiotic Strains
The most commonly used probiotic
preparations are those derived from the lactic acid bacteria (Lactobacillus).
These organisms are found in large numbers in the intestines of healthy
animals.4 Bifidobacterium strains are also probiotic species
typically consumed (Table 1). Other organisms currently available
include bacilli, yeasts, and filamentous fungi. These products are
commercially supplied in the form of powders, capsules, wafers, tablets,
pastes, and sprays.
Prevalence of Eczema
Eczema is
subdivided into atopic and nonatopic eczema.8 Atopic
eczema is a group of diseases involving inflammation of the skin with intense
itching, reddening, dryness, blistering, and scaling.9 The disease
most commonly begins early in infancy and childhood. Infants are prone to
weeping inflammatory patches and crusted areas on the face, neck, extensor
surfaces, and groin.10 The prevalence of atopic eczema varies
between countries. It is estimated that fewer than 2% of children in China and
Iran exhibit atopic eczema, but as many as 20% of children in northern and
western Europe, Australia, and the U.S. exhibit atopic eczema.8
With the incidence of atopic disease rising in Western countries, data have
indicated early immune dysregulation in the infant gut and disruption of the
establishment of normal, healthy gut microflora.11
Why are so many children
affected with eczema? In 1989, Strachan hypothesized that infections in early
childhood acquired from older siblings might confer protection against atopic
diseases, such as atopic eczema.12 This theory became known as the hygiene
hypothesis and suggests that environmental changes in the industrialized
world have led to reduced microbial contact at an early age. Rautava and
colleagues have further studied this topic and concluded that an extended
hygiene hypothesis implies that the epidemic of atopic disease is a result of
altered living conditions and improved hygiene, which are causing drastic
changes in factors affecting the initial establishment of indigenous
intestinal microbiota.12
Clinical Evaluation of
Eczema
In order to evaluate the severity of
atopic disease as objectively as possible, the European Task Force on Atopic
Dermatitis developed a method designed for consistent assessment via an index
called SCORAD (severity scoring of atopic dermatitis). The SCORAD index
was developed in 1993 and evaluates five clinical signs: erythema,
vesiculation, excoriation, crusting, and edema. Each of these signs has four
scoring points, ranging from 0 to 3: 0 = absent; 1 = mild; 2 = moderate; 3 =
severe. The score is determined based on several criteria relating to lesion
spread and intensity, as well as subjective signs. Many clinical trials use
the SCORAD index changes as their end point to evaluate treatment efficacy.
Proposed Mechanism of
Action of Probiotics
Most clinical
studies have shown that probiotics can reduce symptoms in pediatric patients.11
The rationale for its use is based on the potential effects of friendly
bacteria on cellular immune responses. T cells--white blood cells derived from
the thymus gland--fight infection and participate in a variety of cell-mediated
immune reactions. The two main types of T cells are killer (or
cytotoxic), which can kill tumor- and virus-infected cells, and helper,
which provide help to killer T cells, B cells, and macrophages. T helper cells
have a major role in the adaptive immune response. T helper cells are divided
into two subtypes: TH1 and TH2. The TH1
subtype targets organisms that get inside our cells, known as cell-mediated
immunity. The TH2 subtype attacks organisms found outside the
cells in blood and other bodily fluids, known as antibody-mediated immunity.
Accumulating evidence indicates that the overreactivity of TH2
responses is associated with atopic disease.12 It has been
speculated that exposing infants to friendly bacteria early in life helps
mature the TH1 cell immune response and could inhibit development
of the TH2 allergic response, thus decreasing the risk of atopic
disease.13
Healthy microflora in the
intestines may serve as a mucosal barrier against invading organisms.14
It has been proposed that atopic children have an impairment in the
development of their normal gut mucosal barrier. This disruption may lead to
increased antigen transport and high-level antigen exposure during the first
few months of life, which predisposes them to allergic sensitization, thus
resulting in atopic disease.11 Some probiotic strains may
strengthen the lining of the intestines and prevent antigen transport,
decreasing the occurrence of atopic disease. It is also important to note that
stress, illness, antibiotic treatment, changes in diet, or physiological
alterations in the gut may cause the integrity of the microflora to become
impaired.5 Using probiotic agents during stressful times may also
help to maintain intestinal integrity.
Literature suggests that
probiotics help reinforce different lines of gut defense by competing with
pathogens for binding and receptor sites. This is thought to enhance host
resistance to pathogens and aid in their eradication.15 In
addition, probiotics are believed to stimulate production of compounds that
inhibit or destroy pathogens.6
Clinical Trials
Treatment of
Eczema: Several
studies have been conducted regarding the use of probiotics in the treatment
of eczema. One study involved 56 children ages 6 to 18 months with moderate or
severe atopic eczema who participated in a randomized, double-blind,
placebo-controlled trial.15 Children were given a probiotic (1 x 109
Lactobacillus fermentum PCC) or placebo for eight weeks. At the end of
the study, outcomes were measured by the SCORAD index. The reduction in the
SCORAD index over time was significant in the probiotic group (P = .03)
but not in the placebo group. This study concluded that providing
supplementation with probiotic L. fermentum PCC is beneficial in
improving the extent and severity of atopic eczema in young children with
moderate or severe disease.15 This study also concluded that the
positive effects of probiotic supplementation were apparent two months after
supplementation was ceased.15
Another randomized study
assessed the potential of probiotics to control allergic inflammation at an
early age.16 Twenty-seven infants (mean age, 4.6 months) who
exhibited atopic eczema were weaned from breast-feeding and were either given
extensively hydrolyzed whey formulas that were probiotic supplemented with Bifidobacterium
lactis Bb12 or L. GG (LGG) strain or were given the same formula
without probiotics. After eight weeks, a significant improvement in skin
condition was seen in infants given probiotic-supplemented formulas, compared
to the unsupplemented group.16 These data further indicate the
effect probiotics may have on the inflammatory responses.
In a double-blind,
placebo-controlled, crossover study, two probiotic Lactobacillus
strains (L. rhamnosus and L. reuteri) were given in combination
for six weeks to children ages 1 to 13 years with atopic eczema.17
The clinical severity of atopic eczema was evaluated by using the SCORAD
index. Eosinophil cationic proteins in serum and cytokine production were also
measured as inflammatory markers. To assess allergic sensitization, patients
were given a skin prick test. Those who had a positive skin prick test
response and increased IgE levels were classified as allergic patients.
After active probiotic treatment, 56% of the patients experienced improvement
of their eczema, compared to 15% in the placebo group. Although the overall
SCORAD index did not change significantly, the treatment response was more
profound in allergic patients. Thus, the study concluded that a combination of L.
rhamnosus and L. reuteri was beneficial in the management of atopic
eczema.17
Prevention of Eczema:
A double-blind, randomized, placebo-controlled trial was performed to examine
the occurrence of atopic eczema in infants. Mothers who had at least one
first-degree relative (or partner) with atopic eczema, allergic rhinitis, or
asthma were given LGG prenatally. LGG was also given to the infants
postnatally for six months.18 After two years, atopic eczema was
diagnosed in 46 of the 132 (35%) children. The frequency of atopic eczema in
the probiotic group was half that in the placebo group. A follow-up study
after four years showed that 14 of 53 children receiving LGG had developed
atopic eczema, compared to 25 of 54 receiving placebo. Thus, the preventive
effect of LGG on atopic eczema in at-risk children extends to the age of 4
years.18
Safety
To assess the
safety of probiotic ingestion, a randomized double-blind study was conducted
in 118 infants.19 This study evaluated tolerance, effects on
growth, clinical status, and intestinal health. Infants were divided into
three groups. Group A received 1 x 106 colony-forming units (cfu)/g
of B. lactis and Streptococcus thermophilus. Group B received 1
x 107 cfu/g of B. lactis and S. thermophilus. Group C
was an unsupplemented group. Results demonstrated that the supplemented
formulas were well accepted by the infants. Statistically significant
decreases were seen in frequency of colic or irritability (P < .001)
and antibiotic use (P < .001). The study found no difference in growth,
day-care absenteeism, or other health variables. Probiotic use was deemed well
tolerated and safe for infants.19
Probiotic safety has not been
a major area for concern, since fermented foods are often used in our diet.
Probiotic species have been used in food processing for years. In fermented
foods, probiotic bacteria helps produce flavor compounds, such as yogurt and
cheese, improves the nutritional value of food, as in the release of free
amino acids or the synthesis of vitamins, and preserves milk with the
generation of lactic acid and possible antimicrobial compounds.4
There is a long history of safety with probiotic use.
Adverse Effects
Most large clinical studies have not
found any serious problems with probiotic ingestion. In one case, a person
taking LGG developed a liver abscess that was caused by a probiotic strain.
Patients who have suffered complications due to probiotic strains were more
likely to have severe comorbidities or surgeries or were immunosuppressed.
Rare case reports have identified probiotic-related bacteremia and fungemia in
patients with underlying immune compromise, chronic disease, or debilitation.
There have been no reports of bacteremia or fungemia sepsis related to
probiotic use in otherwise healthy people. Most cases linked to probiotic
sepsis have been resolved with appropriate antimicrobial therapy.20
Contraindications include allergies to bacteria or yeast products.
Patient Counseling
Recommended daily
intake for probiotic supplements ranges from one billion units (sometimes
referred to as cfu) to 10 billion units per day.21 These
amounts may be seen on the label as 1 x 109 or 109 for
one billion units and 1 x 1010 or 1010 for 10 billion
units. No specific dosing guidelines are available for children, but most
clinical trials of atopic eczema in children contained probiotic doses ranging
from 1 x 106 to 1 x 1010 cfu/day. In Europe, some infant
formulas contain probiotics. The dose in these formulas can be as high as 1010
cfu/day. When taking antibiotics, it is advisable to take probiotics at least
two hours apart from an antibiotic. The antibiotic could recognize the
probiotic as bacteria and kill the organism. Although not a requirement,
refrigerating probiotic products help prolong their shelf-life.21
Summary
The prevalence of
atopic disease has increased over the past few decades, particularly in
Western societies. Recent research points to early immune dysregulation in the
intestines of infants and the disruption of normal, healthy gut microflora
establishment. Managing atopic eczema in children can be extremely frustrating
for both the parent and child. Treatment options are often expensive and
inconvenient. The growing interest in using probiotic treatment brings hope to
many patients suffering from this disorder. Evidence supports the use of
probiotics for the treatment of atopic eczema, as well as prenatally aiding in
prevention of the disease. Pharmacists should keep in mind that probiotics are
considered a food supplement and are not regulated by the FDA. Finding a
respectable manufacturer is of utmost importance when considering probiotic
formulations. Some Internet resources can provide guidance to unbiased testing
of products currently on the market. Clinical evidence suggests that probiotic
strains appear safe but should probably be avoided in patients who are
immunocompromised. As with any product, pharmacists should consider proper
dosages, storage, and the expiration date when recommending probiotic products
to their patients.
References
1. The Use of Complementary and Alternative Medicine in the United States. Available at: www.nccam.nih.gov/news/camsurvey_fs1.htm. Accessed June 20, 2006.
2. Naturopathic Medicine. CAM Spending. Available at: www.aanmc.org/nat_med/healthcare.php#spending. Accessed June 20, 2006.
3. Probiotics: Ingredients, Supplements, Foods. Available at: www.bccresearch.com. Accessed June 20, 2006.
4. Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Applied Microbiol. 2006;100:1171-1185.
5. Probiotics. Available at: www.freece.com. Accessed May 26, 2006.
6. Young R, Huffman S, et al. Probiotic use in children. J Pediatr Health Care. 2003;17:277-283.
7. Lactobacillus. Available at www.naturaldatabase.com. Accessed October 14, 2005.
8. Brown S, Reynolds NJ. Atopic and non-atopic eczema. BMJ. 2006;332:584-588.
9. The Atopic Eczema/Dermatitis Syndrome. Available at: www.worldallergy.org. Accessed May 30, 2006.
10. Cookson WO, Moffatt MF. The genetics of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2002;2:383-387.
11. Ogden NS, Bielory L. Probiotics: a complementary approach in the treatment and prevention of pediatric atopic disease. Curr Opin Allergy Clin Immunol. 2005;5:179-184.
12. Rautava S, Ruuskanen O, Ouwehand A, et al. The hygiene hypothesis of atopic disease–an extended version. J Pediatric Gastroenterol Nutr. 2004;38:389-388.
13. Murch SH. Probiotics as mainstream allergy therapy? Arch Dis Child. 2005;90;881-882.
14. Macfarlane GT, Cummings JH. Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? BMJ. 1999;318:999-1003.
15. Elmer GW. Probiotics: ‘living drugs.' Am J Health-Systems Pharm. 2001;58:1101-1109.
16. Isolauri E, Arvola T, Sutas Y, et al. Probiotics in the management of atopic eczema. Clin Exp Allergy. 2000;30:1604-1610.
17. Rosenfeldt V, Benfeldt E, Nielsen SD, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol. 2003;111:389-395.
18. Kalliomaki M, Salminen S, Poussa T, et al. Probioitcs and prevention of atopic disease: 4-year follow-up of a randomized placebo-controlled trial. Lancet. 2003;361:1869-1871.
19. Saavedra J, Abi-Hanna A, Moore N, et al. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr. 2004;79:261-267.
20. Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256-1264.
21. Product Review: Probiotic
Supplements and Foods. Available at: www.consumerlab.
com.
Accessed September 8, 2005.
To comment on this article,
contact editor@uspharmacist.com.






