US Pharm. 2010;35(3)(Oncology suppl):4-7.
ABSTRACT: Biologics—preparations created from living organisms by biological processes—have a wide range of uses in oncology. Cancer may be the result of a malfunction of the immune system, and biologic therapy can repair, stimulate, or enhance the immune response. Traditional cancer treatments, although effective, often have significant limitations. Biologics can target the cancer in a specific way and may work synergistically with chemotherapy to improve outcome. Since biologics utilize the immune system, it may be advantageous to use them before the immune system is compromised. The high cost of these products may limit their use.
Biologics—including drugs, vaccines, and antitoxins—are preparations that are synthesized from living organisms or their products and used as diagnostic, preventive, or therapeutic agents. The first substance approved for therapeutic use was biosynthetic “human” insulin made by recombinant DNA technology in 1982. Since then, biologics have had a profound effect on rheumatology, dermatology, gastroenterology, oncology, and other medical disciplines. In oncology, therapeutic biologic products are used for the prevention and treatment of various types of cancer, as well as to treat symptoms related to cancer therapy. TABLE 1 summarizes some of the biologic agents that pharmacists may encounter in patients being treated for cancer.

Biologic therapy is designed to repair, stimulate, or enhance the immune response; this can be achieved with great specificity. In many cases, conventional treatments for cancer—such as surgery, radiation, and chemotherapy—have clinical success, but they also have limitations. Surgery and radiotherapy are effective for the treatment of localized tumors, but they may not be useful for disseminated disease or for tumors located in areas that are difficult or dangerous to reach. Traditional chemotherapy, although effective, can be severely toxic to normal tissue. Despite the potency of cytotoxic chemotherapy and the specificity of immunotherapy, neither method alone has been sufficient to eradicate the disease. However, there is evidence that standard chemotherapy may work in synergy with active immunization for more effective antitumor immunity, and there is great potential for the combination of these treatment modalities.1
The identification of tumor-specific antigens, lymphocytes, and T-cell responses in cancer patients led to immunotherapies designed to enhance antitumor immune response.1 T cells are potent cellular effectors of the immune system and have a memory that responds if rechallenged by the same antigen.2 Since tumor immune therapy is more specific and less toxic than traditional chemotherapy, it may be an ideal adjuvant treatment for patients with malignancies that have a high risk of relapse. Chemotherapy could be used first to induce the active disease into remission, followed by tumor immune therapy via memory T cells.2 Immunotherapies seem to be effective against small tumor burdens, but they are not capable of controlling large tumor masses. Moreover, patients with advanced disease have compromised immunity, which limits the effectiveness of immunotherapy. Therefore, it has been proposed that immunotherapy be used in the early stages of tumorigenesis.1
BIOLOGIC AGENTS
Cytokines
Cytokines are proteins that enhance or suppress the immune system by either attacking foreign, infected, or damaged cells or signaling other cells to attack.3 Classes of cytokines include interferons, interleukins (ILs), colony-stimulating factors (CSFs; see discussion in Growth Factors), and monoclonal antibodies (MABs).
MABs: MAB therapy is a form of passive immunity that uses genetically engineered antibodies directed against antigens on the surface of tumor cells.3 Originally, MABs were made entirely from mouse cells, but now some or all of the antibody proteins are human, which leads to increased effectiveness and a decreased chance of serious side effects.
Since many MABs are developed from cells originating in other species, infusion reactions are common. MABs are usually administered via intravenous infusion and are often given in an ambulatory care clinic. Premedication with acetaminophen, diphenhydramine, and sometimes a histamine2 receptor agonist is standard. Typically, side effects are mild and similar to an allergic reaction. Patients should not receive vaccinations with live viruses during treatment. In addition, pharmacists may need to be aware of special instructions related to storage, handling, and administration of these products (TABLE 2).
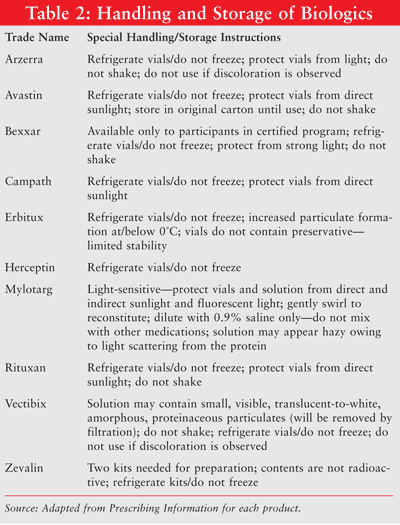
Side effects of MABs include allergic reactions (hives, itching); flulike symptoms (headache, malaise, fever, chills); dyspnea and mild wheezing; and gastrointestinal symptoms (nausea, vomiting, diarrhea). Other side effects that may occur are skin rash, tachycardia, hypotension, and neutropenia.
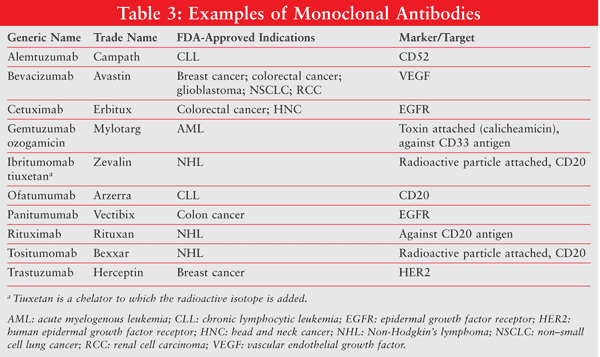
MABs work by various mechanisms. They can mark the cancer cell to make it more visible to the immune system by attaching to specific proteins found on B cells or T cells (i.e., CD20, CD52 antigens).3 They can block growth-factor receptors and inhibit the growth of new cells. Epidermal growth-factor receptor is one receptor that accepts growth signals; it is expressed on the cell surface of normal cells and cancer cells. Human epidermal growth factor receptor (HER) binds to cell-surface receptors and regulates normal cell proliferation and survival. There are four known HER proteins: HER1, -2, -3, and -4. Vascular endothelial growth factor inhibits the ability of tumors to form new blood vessels to feed their growth.3 When a radioactive particle is combined with an MAB, radiation is delivered directly to cancer cells. Radiation-linked MABs deliver a low level of radiation over a longer period of time and can be as effective as high-dose external beam radiation.4 For example, the MAB ibritumomab attaches to receptors on cancerous blood cells to deliver radiation. Chemotherapy agents, toxins, or other molecules also can be attached to MABs. The attached molecules are inactive until they have reached the target cell, where they are activated, causing cell death and minimizing harm to healthy cells.5
TABLE 3 lists some of the currently approved MABs for the treatment of cancer, along with the target or mechanism.
Interferons: Interferons are proteins made and released in response to the presence of pathogens, including viruses, bacteria, and tumor cells. They allow communication between cells to trigger the immune system to destroy invading cells.6 Interferon alfa is used to treat hairy-cell leukemia, melanoma, follicular non-Hodgkin’s lymphoma, and Kaposi’s sarcoma. Interferon alfa-2a, recombinant (Roferon-A; Hoffmann-La Roche) and interferon alfa-2b, recombinant (Intron A; Schering-Plough) are examples of interferons available in the United States.
ILs: IL-2 is a protein that activates the immune system to regulate inflammatory and immune responses. One IL-2 used in cancer treatment is aldesleukin (Proleukin; Novartis), which is indicated for the treatment of metastatic renal cell carcinoma and metastatic melanoma.
Growth Factors
Hematopoietic growth factors (HGFs) are a group of substances with the ability to support hematopoietic colony formation in vitro.7 This group of substances includes erythropoietin, IL-3, and CSFs. HGFs are used to promote bone marrow proliferation in neutropenia following cytotoxic chemotherapy or a bone marrow transplant.
Granulocyte colony-stimulating factor (G-CSF) is a blood growth factor that stimulates the bone marrow to produce neutrophils. Chemotherapy can damage rapidly dividing cells such as hair follicle cells and bone marrow cells. G-CSFs are used to increase the number of neutrophils and subsequently reduce the risk of infection. These products are also used in patients who receive a bone marrow transplant and for other myelodysplastic syndromes. Filgrastim (Neupogen; Amgen) is an example of a G-CSF.
A growth factor–toxin combination currently approved by the FDA is denileukin diftitox (Ontak; Eisai).8 It attaches the growth factor IL-2 to a toxin from the microorganism that causes diphtheria. Denileukin diftitox is used to treat primary cutaneous T-cell lymphoma.
Vaccines
Vaccines, which typically target infectious agents, are given before actual exposure to the disease and prepare the immune system to prevent infection. New vaccines against human papillomavirus work in a similar way to prevent cervical, vaginal, and vulvar cancer.9 Vaccines against hepatitis B virus may lower the risk of liver cancer.
Although vaccines for the treatment or prevention of cancer have been extensively studied and some show promise in clinical trials, none have been approved in the U.S. for these indications. The goal of a cancer vaccine is to seek out and destroy cancer cells and prevent the development of disease. Most experimental cancer vaccines have been designed to stimulate a cellular immune response against antigens from established tumors.10
COST OF BIOLOGICS FOR CANCER THERAPY
There is no question that biologics have been a pioneering step in the treatment of cancer and that the introduction of these compounds has made a significant impact. However, owing to the escalating cost of research and development, along with extremely sensitive and difficult manufacturing processes, biologics are much more expensive to produce than small, simple-molecule products. On average, it costs $1.2 billion to bring a new biologic agent to market.11
These drugs raise questions about cost-effectiveness, especially if they prolong life for only a few months. Health care insurers in the U.S. are concerned about how they are going to pay for them, especially since the number of potential patients is enormous. These products are approved for the treatment of colorectal, lung, and breast cancer, which are the most commonly occurring cancers and account for 38% of all cases in the U.S. annually.11 Moreover, biologics are being investigated for treatment at earlier disease stages and for rare malignancies. In addition, cancer predominantly affects the elderly, a large demographic that will increase in the coming years. Furthermore, even patients with health care coverage will need to pay a substantial portion of the cost, typically around 20% of the price of the drug. For many families and for people on a fixed income, this could be financially devastating, and some patients may decline treatment for financial reasons.
Lawmakers, legislators, and others currently drafting legislation in the U.S. are trying to develop strategies that will allow for a reasonable profit in an effort not to stifle the pace of innovation in cancer research, yet minimize costs by controlling the price of the biologic and/or how it is used.12
Currently, no procedure is in place for the FDA to approve generic versions of biologics, often called biosimilars or follow-on biologics (FOBs). The Federal Trade Commission recently released a report suggesting that giving the FDA the authority to approve FOBs would be an efficient way to bring them to market.13 The report states that competition by FOBs is unlikely to be similar to the competition that exists for traditional, small-molecule, brand-versus-generic drugs (TABLE 4).

CONCLUSION
Primary tumors usually can be treated with a combination of standard therapies; however, preventing metastasis through disseminated tumor cells is not always possible. The goals of cancer immunotherapy are to prevent the disease from spreading and to improve the patient’s quality of life. Recent studies have suggested that there may be synergy between immunotherapy and standard chemotherapy; therefore, it may be beneficial to start biologic therapy earlier in the cancer treatment, before the immune system is compromised.
Although the side-effect profile of biologic agents is relatively mild compared with traditional chemotherapy, biologics often cost much more. Pharmacists should be aware of special storage, preparation, and handling instructions. Moreover, pharmacists should counsel patients about possible side effects, storage issues, and administration techniques for medications that are self-administered.
References
1. Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2009;58:317-324.
2. Chiriva-Internati M, Grizzi F, Wachtel MS, et al. Biological treatment for liver tumor and new potential biomarkers. Dig Dis Sci. 2008;53:836-843.
3. Schuster M, Nechansky A, Kircheis R. Cancer immunotherapy. Biotechnol J. 2006;1:138-147.
4. Gregory SA, Hohloch K, Gisselbrecht C, et al. Harnessing the energy: development of radioimmunotherapy for patients with non-Hodgkin’s lymphoma. Oncologist.2009;14(suppl 2):4-16.
5. Sharkey RM, Goldenberg DM. Targeted therapy of cancer: new prospects for antibodies and immunoconjugates. CA Cancer J Clin. 2006;56:226-243.
6. Aguilar Rebolledo F. Interferons in neurology. Rev Invest Clin. 2000;52:665-679.
7. Vose JM, Armitage JO. Clinical applications of hematopoietic growth factors. Clin Oncol.
8. Manoukian G, Hagemeister F. Denileukin diftitox: a novel immunotoxin. Expert Opin Biol Ther.
9. Schmiedeskamp MR, Kockler DR. Human papillomavirus vaccines. Ann Pharmacother.
10. Mittendorf EA, Peoples GE, Singletary SE. Breast cancer vaccines: promise for the future or pipe dream? Cancer. 2007;110:1677-1686.11. Malik NN. Controlling the cost of innovative cancer therapeutics. Nat Rev Clin Oncol. 2009;6:550-552.
12. Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med.
13. Federal Trade Commission. Providing the FDA with authority to approve follow-on biologics would be an efficient way to bring them to market, lowering consumers’ health care costs. www.ftc.gov/opa/2009/06/biologics.shtm. Accessed February 3, 2010.
To comment on this article, contact rdavidson@uspharmacist.com.





