US Pharm. 2016;41(6):35-39.
ABSTRACT: Autoimmune hepatitis (AIH) is a chronic disease of unknown cause with persistent inflammation of the liver and the potential for necrosis and progression to cirrhosis associated with it. AIH can affect any ethnicity, and it appears to occur in women more frequently than in men. There is not one specific trigger that is associated with the disease. Standard treatment for AIH is a corticosteroid (prednisone) alone or in combination with azathioprine. Alternative therapies for patients who may have suboptimal response to the standard treatment options are also available. An immunosuppressive agent should be initiated as soon as possible after diagnosis to prevent further progression of the disease. Patients who fail to respond to standard treatment or the alternative therapies may develop end-stage liver disease requiring liver transplantation.
Autoimmune hepatitis (AIH) was first reported in 1950 by Swedish physician Jan Waldenström and is defined as the unresolving inflammation of the liver of an unknown cause.1,2 Environmental triggers, immune system failure, and genetics can induce a T-cell–mediated immune attack upon liver antigens, leading to the development of AIH.2 While AIH has been seen in all ages and ethnic groups, it appears to affect women more frequently than men. AIH should be considered in the differential diagnosis of a patient who presents with abnormal serum globulins, one or more characteristic autoantibodies, and elevations in aminotransferases as long as other causes of hepatitis cannot be excluded.2
Background
There are two known types of AIH: type 1 (AIH-1), positive for antinuclear antibodies (ANA) and/or anti–smooth muscle antibody (ASMA), and type 2 (AIH-2), which is positive for anti-liver/kidney microsomal type 1 antibody (anti-LKM-1) or for anti-liver cytosol type 1 antibody (anti-LC-1).1 The CDC does not report epidemiologic data for the prevalence of AIH in the United States. However, to prevent and control hepatitis C virus (HCV) infection and HCV-related chronic disease, the CDC recommends a one-time HCV testing for people born between 1945 and 1965.3 AIH can coexist with other liver diseases and can be triggered by certain viral infections or pharmacologic agents.4
The current standard of therapy of AIH is the use of prednisone alone or in combination with azathioprine to improve the symptoms and manifestations of liver inflammation and to reduce the progression of liver fibrosis.5 Lately, there has been much discussion on the use of alternative therapies in the AIH patient population not only because of the systemic adverse effects of prednisone use, but also because of treatment failure, incomplete response, and drug toxicities experienced by patients. These alternative therapies include mycophenolate mofetil (MMF), tacrolimus, cyclosporine, budesonide, and allopurinol.5 Rituximab, infliximab, and cyclophosphamide have been studied, but limited data exist to support their use in AIH.5 Recent literature concerning the clinical significance of these alternative agents will be discussed.
Clinical Presentation
AIH was originally described in young females, and while women still make up a majority of cases, it is now well known that the condition can occur at any age, in both sexes, and in all ethnic groups.1-5 However, an overall bimodal age pattern has been reported at presentation, one peak during childhood and the teen years and another in middle age between the fourth and sixth decades of life. However, more recent studies have shown that an increasing number of AIH patients are also diagnosed at older ages.6 The clinical presentation of AIH varies from no symptoms to severe acute hepatitis and even fulminant hepatic failure. Approximately 34% to 45% of patients have no symptoms, and about 70% become symptomatic and thus need to be monitored.2 Additionally, about 25% to 34% of patients present with asymptomatic liver test abnormalities. Furthermore, 40% of patients may present with an acute onset, where immunoglobulin G (IgG) levels are normal and ANA are not detected; however, the presentation of severe fulminant hepatic failure is rare and appears to be more common in AIH-2.7
The common clinical presentation of AIH is characterized by one or more of the following nonspecific symptoms of varying severity: fatigue, general ill health, mild pain in the right upper quadrant, lethargy, malaise, anorexia, weight loss, nausea, pruritus, jaundice, and arthralgia involving the small joints; amenorrhea is also common, whereas maculopapular skin rash and unexplained fever are rare features.6 These symptoms are nonspecific and ultimately contribute to the delay in diagnosis. Clinical manifestations may also vary by ethnicity.
Occasionally, AIH may present in some special conditions, including during pregnancy or in the early postpartum period, after administration of certain drugs, following a viral infection, post liver transplantation, and sometimes in the presence of other autoimmune or immune-mediated diseases in patients or their first-degree relatives.6
Diagnosis and Management
Diagnosis: A scoring system, developed by the International Autoimmune Hepatitis Group, is used to diagnose patients with AIH. The patient is given a “definite” or “probable” diagnosis based on certain clinical, laboratory, and histologic criteria. As this is an autoimmune disorder, the presence and level of autoantibody expression by indirect immunofluorescence, serum IgG concentration, compatible or typical histologic features, and the absence of viral markers are used to diagnose patients.2 Conventional autoantibodies of AIH are also assessed, such as ANA, ASMA, anti-LKM-1, and anti-LC-1.2 Key laboratory markers used to assess hepatic function, such as serum alanine (ALT) or aspartate (AST) aminotransferase, alkaline phosphatase (AP), albumin, total or gamma globulin, IgG, and bilirubin (conjugated and unconjugated) are also used to diagnose AIH.2 A definite or probable diagnosis is determined based on the magnitude of these clinical, laboratory, and histologic criteria as well as by patient-specific factors such as history of alcohol exposure, medication history, and infections that could cause liver injury.2
Management: Patients who present with serum AST or ALT levels >10-fold the upper normal limit (UNL); at least fivefold the UNL along with serum gamma-globulin level at least twofold the UNL and/or histologic features of bridging necrosis or multilobular necrosis; and incapacitating symptoms are considered to have significant disease and are eligible to be initiated on immunosuppressive therapy.2,8 In patients with mild disease (i.e., those patients who do not meet the treatment criteria), the risk versus benefit of treatment should be reviewed and the patient referred to a physician.2,8 Asymptomatic individuals with minimal or no disease activity or inactive cirrhosis should continue to be monitored closely for 3 to 6 months for signs of disease progression, as there are little data to support treatment use in these patients at this time.2,8
Standard Therapy
The standard therapy for AIH has been prednisone administered alone or in combination with azathioprine. Details regarding therapies are provided in TABLE 1.2 Induction therapy of prednisone dosages of 30 to 60 mg/day or up to 1 mg/kg/day as monotherapy and a dosage of 30 mg/day combined with azathioprine 50 mg (1-2 mg/kg/day) have been used in patients. Azathioprine cannot induce remission when used on its own, but it allows the maintenance of remission in association with a reduced dose of steroids.1 Azathioprine is introduced in these patients later, so that a treatment response to steroid monotherapy can be evaluated first. Special caution should be taken with patients with comorbid conditions that can occur as a result of the use of systemic prednisone (i.e., vertebral compression, psychosis, brittle diabetes, uncontrolled hypertension). An alternative corticosteroid, budesonide, with fewer steroid-specific adverse effects can be used in combination with azathioprine.5 Before initiating azathioprine, a pretreatment CBC should be done to ensure that the patient does not present with a severe pretreatment cytopenia (below 2.5 × 109/L) or platelet counts below 50 × 109/L.2 Patients should also be screened to ensure that there is no known complete deficiency of thiopurine methyltransferase activity to contraindicate the use of azathioprine.2 Upon remission, prednisone should be tapered gradually over a 6-week period, with laboratory values measured every 3 weeks during therapy and for 3 months after withdrawing.2
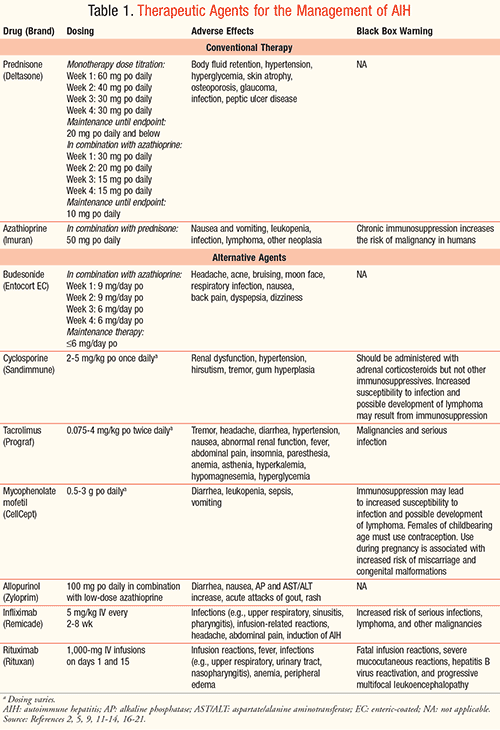
Alternative Agents
There are various alternative agents available in the treatment of AIH (TABLE 1), including budesonide, cyclosporine, tacrolimus, MMF, the adjunct therapy allopurinol, infliximab, rituximab, and ursodeoxycholic acid.9-18 Budesonide, a synthetic steroid, can be used as an alternative to prednisone. Compared to prednisone, it has a relatively safe adverse-effect profile with headache and respiratory infection seen most commonly.9 Cosmetic changes such as moon face, acne, and hirsutism have been noted.8 Budesonide is primarily metabolized through hepatic first-pass metabolism, so patients do not experience as many of the systemic adverse effects seen with prednisone use. However, this metabolism does limit its use in cirrhotic patients due to the risk of portal hypertension, which leads to poor metabolism and the risk of systemic toxicity.
In a large, prospective, multicenter phase II study, 207 patients were randomized to budesonide 3 mg po tid or prednisone 40 mg/day (with a tapering course) plus azathioprine 1 to 2 mg/kg/day.10 Complete response rate was significantly higher in the budesonide group compared to the prednisone group (84% vs. 18%; P < .0001), biochemical remission was superior for budesonide as well (60% vs. 39%; P = .0012), and adverse effects at 12 months were significantly lower in the budesonide group.10 These results show that budesonide is a relatively safe and effective alternative to the standard therapy.
Cyclosporine, a calcineurin inhibitor and immunosuppressive agent, works on calcium-dependent signaling and inhibits T-cell function via the interleukin 2 (IL2) gene. Two case studies involving the use of cyclosporine have shown response rates of 79% and 80%.10 Doses ranged from 2 to 5 mg/kg, but the small sample size warrants further clinical trials to determine cyclosporine’s place in therapy as another option for those who do not achieve remission with standard therapy. Reported adverse effects included hyperkalemia, hypertension, renal failure, hyperlipidemia, gingival hyperplasia, hirsutism, infection, and malignancy.11 There are many significant drug-drug interactions with cyclosporine, as it is extensively metabolized by CYP3A4 and is a substrate of P-glycoprotein (Pgp). To avoid any potential toxicity, it is important to review the patient’s medication history prior to initiating therapy.
Tacrolimus, a macrolide immunosuppressive agent, works by inhibiting T-cell activation. Although no controlled trials currently exist for its use in AIH patients, the results from pilot studies are encouraging and further study in clinical trials is warranted in order to recommend tacrolimus as a safe and effective agent.10 Adverse effects noted with tacrolimus are infection, tremors, hypertension, abnormal renal function, peripheral edema, alopecia, constipation, diarrhea, and nausea and vomiting.12 Tacrolimus is metabolized by the CYP3A4 pathway, and dosing adjustments would be required in patients taking concomitant medications that are strong inhibitors and inducers of CYP3A4.
MMF is known for its use as an immunosuppressive agent in organ transplantation. It is a prodrug that is converted in the liver to the active metabolite mycophenolic acid. The small number of clinical trials including MMF in patients with AIH limits its use in a larger patient population.10,13 A retrospective, observational study was conducted in the United Kingdom in 20 patients diagnosed with AIH from January 2000 to May 2010.13 Nonresponders to steroid therapy or azathioprine were initiated on MMF 500 mg twice daily for 2 weeks, and if the dose was tolerated, it was increased to 1 g twice daily. After a follow-up period of 47 months, 14 of the 20 patients were still on MMF with biochemical remission, including 4 out of 5 patients with cirrhosis. Patients need to be educated on the potential risks with taking MMF, including the risk of infection, development of lymphoma, and pregnancy loss and congenital defects. The need for laboratory monitoring in patients during the course of therapy should be noted as well.14 Females of reproductive age taking MMF must use two forms of contraception for 4 weeks before starting therapy, during the entire course of therapy, and for 6 weeks after stopping.14
Allopurinol, known mostly for its use in treating gout, is another agent that has shown success in a specific AIH patient population in clinical trials. Approximately 10% of patients with AIH are nonresponsive or intolerant to thiopurine therapy, which can lead to the formation of the hepatotoxic thiopurine metabolite (6-methyl-mercaptopurine) instead of the active metabolite 6-thioguanine nucleotides (6-TGNs).15 Concomitant use of allopurinol and low-dose azathioprine can prevent the formation of the toxic metabolite. A clinical trial conducted at the VU University Medical Center in Amsterdam from February 2011 through October 2012 initiated this combination in eight patients with AIH.15 Patients were switched to allopurinol 100 mg and low-dose thiopurine (azathioprine 75-150 mg) combination therapy because they showed a nonresponse or loss of response on conventional thiopurine doses after laboratory values showed increased ALT levels.15 The clinical benefit showed a sustained reduction and normalization of ALT levels in seven of the eight patients, and there were no major drug-related adverse effects noted. However, patients starting allopurinol should be informed of the potential side effects of the drug and be monitored routinely while on therapy. The most common effects include diarrhea, nausea, increased liver enzyme tests (AP and AST/ALT), acute gout attacks, and skin rash.16
Three additional agents have shown some promise in managing patients with difficult-to-treat AIH. Infliximab, a monoclonal antibody against antitumor necrosis factor-alpha, has been studied. There is caution against its use because it is associated with the induction of severe de novo AIH in some patients treated for other diseases.1 Rituximab, an anti–B-cell monoclonal antibody against the protein CD20 has also been used in AIH patients. Both infliximab and rituximab put patients at risk for severe complications, so patients must be carefully monitored while receiving treatment (TABLE 1). Ursodeoxycholic acid has also been studied, but its efficacy has not yet been shown in AIH.1 These three agents need to be studied more in the AIH population to determine their place in therapy.
Immunosuppressive therapy is continued in AIH patients until a treatment endpoint is reached, such as remission, treatment failure, incomplete response, or drug toxicity. Patients are carefully and routinely monitored to ensure that therapy is working and to intervene when signs of treatment failure become known to avoid further liver function decline. A case of AIH is in true remission when symptoms disappear; laboratory tests show normal serum aminotransferases, bilirubin, and gamma globulin levels; and histologic studies show normal hepatic tissue or inactive cirrhosis.2 Treatment failure is defined as worsening of disease despite medication adherence, and patients can develop jaundice, ascites, or hepatic encephalopathy. An incomplete response to therapy would show some or no improvement in clinical, laboratory, and histologic features despite medication adherence, sometimes years after therapy has been completed. In the event of drug toxicity, the patient may experience intolerable adverse effects, and the dose will need to be reduced slowly.2 Upon discontinuation, the patient should be maintained on an alternative agent that is tolerated.2
Conclusion
From the information presented, it is clear that the use of alternative therapies in the AIH patient population can be considered in patients who are unable to tolerate or are unsuccessful with the standard prednisone regimxen. Practitioners now have the ability to individualize therapy to fit the therapeutic needs of specific AIH patient populations. There are currently limited clinical data to recommend the use of cyclosporine, tacrolimus, MMF, allopurinol, infliximab, and rituximab for a larger patient population. With the increased use of these alternative agents to treat AIH, there are more opportunities for avoiding treatment failure, incomplete response, and drug toxicity in the future.
REFERENCES
1. Liberal R, Grant CR, Mieli-Vergani G, Diego Vergani D. Autoimmune hepatitis: a comprehensive review. J Autoimmun. 2013;41:126-139.
2. Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):1-31.
3. Smith BD, Morgan Rl, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
4. Wolf DC. Autoimmune hepatitis: practice essentials, background, pathophysiology. Medscape. December 28, 2015. http://emedicine.medscape.com/article/172356-overview#a3. Accessed January 18, 2016.
5. Manns MP, Taubert R. Treatment of autoimmune hepatitis. Clin Liver Dis. 2014;3(1):15-17.
6. Zachou K, Muratori P, Koukoulis GK, et al. Review article: autoimmune hepatitis—current management and challenges. Ailment Pharmacol Ther. 2013;38(8):887-913.
7. Makol A, Watt KD, Chowdhary VR. Auto-immune hepatitis: a review of current diagnosis and treatment. Hepat Res Treat. 2011;2011:390916.
8. Fialho A, Fialho A, Carey WD. Autoimmune hepatitis. Cleveland Clinic Center for Continuing Education; July 2015. www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hepatology/chronic-autoimmune-hepatitis/#top. Accessed January 25, 2016.
9. Entocort EC (budesonide) package insert. Södertälje, Sweden: AstraZeneca; 2009. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021324s008lbl.pdf. Accessed January 26, 2016.
10. Floreani A, Liberal R, Vergani D, Mieli-Vergani G. Autoimmune hepatitis: contrasts and comparisons in children and adults—a comprehensive review. J Autoimmun. 2013;46:7-16.
11. Sandimmune (cyclsporine) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; March 2015. www.pharma.us.novartis.com/product/pi/pdf/sandimmune.pdf. Accessed January 26, 2016.
12. Prograf (tacrolimus) package insert. North-brook, IL: Astellas Pharma US, Inc; May 2015. www.astellas.us/docs/prograf.pdf. Accessed January 26, 2016.
13. Jothimani D, Cramp ME, Cross TJ. Role of mycophenolate mofetil for treatment of autoimmune hepatitis—an observational study. J Clin Exp Hepatol. 2014;4(3):221-225.
14. CellCept (mycophenolate mofetil) package insert. South San Francisco, CA: Genentech USA, Inc; July 2015. www.gene.com/download/pdf/cellcept_prescribing.pdf. Accessed January 26, 2016.
15. de Boer YS, Van Gerven NM, de Boer NK, et al. Allopurinol safely and effectively optimises thiopurine metabolites in patients with autoimmune hepatitis. Aliment Pharmacol Ther. 2013;37(6):640-646.
16. Zyloprim (allopurinol) product information. San Diego, CA: Prometheus Laboratories Inc; November 2009. http://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=342832b5-1a32-4bea-bc49-ab0fd152154e&type=display. Accessed January 26, 2016.
17. Micromedex 2.0. Greenwood Village, CO: Truven Health Analytics, Inc. www.micromedexsolutions.com. Accessed January 25, 2016.
18. Imuran (azathioprine) package insert. San Diego, CA: Prometheus Laboratories Inc; May 2011. www.accessdata.fda.gov/drugsatfda_docs/label/2011/016324s034s035lbl.pdf. Accessed January 26, 2016.
19. Remicade (infliximab) package insert. Horsham, PA: Janssen Biotech, Inc; October 2015. www.remicade.com/shared/product/remicade/prescribing-information.pdf. Accessed May 9, 2016.
20. Rituxan (rituximab) package insert. San Francisco, CA: Genentech, Inc; April 2016. www.gene.com/download/pdf/rituxan_prescribing.pdf. Accessed May 9, 2016.
21. Moura M, Liberal R, Cardoso H. Management of autoimmune hepatitis: focus on pharmacologic treatments beyond corticosteroids. World J Hepatol. 2014;6(6):410-418.
To comment on this article, contact rdavidson@uspharmacist.com.






