US Pharm. 2015;40(3):35-38.
ABSTRACT: Pain management is complicated and is becoming more difficult, especially as pain medications are being more strictly controlled. An increasingly popular pain management strategy is the use of noncontrolled topical pain management agents, including diclofenac and lidocaine. These agents are not appropriate for managing all types of pain, but they are a good option for select states of chronic pain. It is important for pharmacists to be aware of the available options in order to contribute to the care of patients with chronic pain.
The rate of death due to overdose of pain medications is rising significantly, with an increase of 400% in women and 265% in men over a 10-year period in the United States.1 When the Drug Enforcement Administration (DEA) announced in August 2014 the rescheduling of hydrocodone/acetaminophen (Vicodin) from a Schedule III (CIII) to a Schedule II (CII) drug, the world of primary care and chronic pain management was thrown into a tailspin.2 Before the rule became effective in October 2014, there were reports of fraudulent Vicodin prescriptions being called in to pharmacies. Concerns over documentation of CIIs began to arise in physicians’ offices, as this change would increase demands on their staff and inconvenience patients.
With the rescheduling of one of the most commonly prescribed prescription pain medications, the increased burden on both prescribers and patients has forced prescribers to be creative in the management of pain. With this, a heightened awareness of topical pain medications has developed. Prescription options for nonopioid topical analgesia in the U.S. include diclofenac and lidocaine.
CLASSIFICATION OF PAIN
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”3 Chronic pain affects about 100 million Americans and accrues to an annual healthcare cost of approximately $600 billion in the U.S.4
There are many ways to classify pain, aside from designating it as acute or chronic.3 Classification of pain is important to ensure that treatment is appropriate.5 Chronic pain is generally understood to be pain that remains beyond the expected healing time, which is typically between 3 and 6 months.5 The location of the pain may be generalized, affecting the entire body, or localized, affecting a specific site, such as the elbow.5
There are two sources from which pain originates: somatic and visceral.3,6 Localized, throbbing pain that originates from damage to the skin, musculoskeletal system, or joints is considered to be somatic.3,6 Visceral pain is referred or diffuse pain originating from internal organs, and it carries a greater emotional burden.3,6
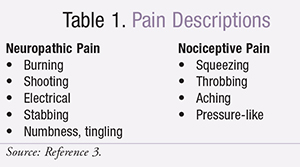
When looking at the etiology of pain, it can be categorized as nociceptive, neuropathic, or of mixed etiology (TABLE 1).3,5,6 Pain resulting from damage to a somatic source (e.g., musculoskeletal) is considered to be nociceptive pain.3,5,6 When pain results from damage to the nervous system (peripheral or central), it is classified as neuropathic (e.g., postherpetic neuralgia).3,5,6 When a patient experiences a combination of nociceptive and neuropathic pain, it is categorized as being of mixed etiology.5
![]()
PAIN INTENSITY
The intensity of pain is easy to assess in any practice setting. The most widely used measurement tools include the numerical rating scales and visual analogue scales.3 The visual analogue scale uses a 10-cm line with one end representing no pain and the other end representing the worst possible pain.3 The numerical rating scale asks patients to rate their pain on a scale from 0 to 10, with 0 being no pain and 10 being the worst possible pain.3 A score of 1 to 3 is considered mild pain, while a score of 4 to 5 is considered moderate pain.3 Any score of 6 or greater on the numerical rating scale is considered to be severe pain.3
Topical therapies are not appropriate for all states of chronic pain, but they do play a role in the management of chronic musculoskeletal pain, peripheral neuropathic pain, and pain in the cancer and palliative care setting.5,7
BENEFITS OF TOPICAL FORMULATIONS
While oral pain medications have long been the mainstay of therapy, they are not without risk.5 The systemic absorption of these medications puts patients at risk for serious adverse events.8 In the hope of producing similar efficacy while reducing the risk for adverse events, alternate formulations have been developed, including buccal, sublingual, topical, transdermal, rectal, intranasal, subcutaneous, and IV.8 To reduce the risk of systemic adverse events, topical administration via topical patches, gels, creams, ointments, and solutions aims to provide local analgesia with a lesser degree of systemic distribution.8,9 Transdermal patches were developed to deliver systemic analgesia, while bypassing major organ systems known to react adversely to the drug.8,9 For example, the goal of transdermal fentanyl is to produce systemic analgesia while bypassing absorption in the gastrointestinal (GI) system. This reduces the likelihood of opioid-induced nausea, vomiting, and diarrhea.8
Topical medications must be able to penetrate and diffuse readily into the targeted tissue.8 To pass the corneum stratum, lipophilic, hydrophilic, and low-molecular-weight characteristics are desired.8 Because topically administered products avoid absorption in the GI tract and have limited first-pass metabolism, they have significantly less systemic exposure when compared to orally administered medications, despite effective concentrations in the targeted tissues.8
DICLOFENAC SODIUM
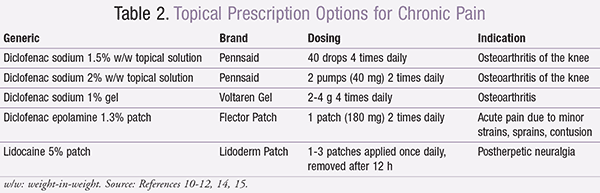
Diclofenac is a nonsteroidal anti-inflammatory drug (NSAID) that exhibits its action via cyclooxygenase (COX) enzyme inhibition.8,10 Inhibition of these enzymes triggers a reduction in prostaglandins and thromboxanes, which regulate the inflammatory process.8,10 Diclofenac has higher selectivity for inhibition of COX-2, which is believed to have an increased role in the regulation of the inflammatory process. COX-2 selective NSAIDs have been linked to cardiovascular thrombotic risk, due to the presence of COX-2 in the cardiovascular tissues.8,10 The black box warnings of increased cardiovascular and GI risk are present in the prescribing information for all dosage forms of diclofenac.10,11 Topical diclofenac is available in a solution, gel, and patch.10,11
Diclofenac Sodium 1% Gel
Diclofenac sodium 1% gel is dispensed in tubes containing 100 g each.10 It can be prescribed in a 3- or 5-tube package and should be stored at room temperature.10 Each prescription should be administered with a dosing card, which allows the patient to measure the correct amount of 2 or 4 g. Treatment of the elbow, wrist, or hand warrants the 2-g dose four times daily, while 4 g should be applied four times daily to the knee, ankle, or feet. The dosing card can be used to administer the doses, after which it should be rinsed and dried prior to storage for the next use. The gel should be rubbed into the skin. No more than 16 g should be applied to any one lower extremity or 8 g to any one upper extremity joint per day. The maximum amount per day for all areas should not exceed 32 g. After application to the hand or wrist, 1 hour should be allowed to lapse prior to hand washing, as it should for bathing after application to any joint. Neither heat nor occlusive dressing should be applied post application of diclofenac sodium 1% gel.10
Diclofenac 1.3% Patch
The diclofenac patch is available in a box of 6 envelopes, each containing 5 patches.11 Each patch is 10 cm by 14 cm in size and contains 180 mg of diclofenac epolamine. The patches should be stored at room temperature, and the envelopes should remain sealed when not in use. One patch is to be applied twice a day to the most painful area of intact, undamaged skin. The patch should not be worn during bathing, as this reduces adhesiveness.11 Tape can be used on the edges of the patch for reinforcement, should it start to peel.11 If that does not solve the problem, a mesh sleeve or netting may be used. It is important that this type of covering be used because of its nonocclusive nature. Heat and occlusive coverings should not be used due to the risk of increased absorption of the active ingredient. Patients should be instructed to wash their hands after applying, touching, or removing the diclofenac patch.11
Diclofenac Sodium Topical Solution
Diclofenac sodium solution is specifically indicated to treat pain associated with osteoarthritis of the knee. It is available in two strengths, 1.5% and 2%.12-14
1.5% Solution: Diclofenac 1.5% solution comes packaged in either 60- or 120-mL bottles with a dropper.12,13 Each mL of solution contains 16.05 mg of active ingredient. For pain relief, patients are instructed to apply 40 drops of solution to each painful knee four times daily. It is recommended that 10 drops be applied at one time and then rubbed in to avoid spillage of the medication. The solution should be dispersed to the front, back, and sides of the knee during administration.12,13
2% Solution: Diclofenac 2% solution is available in a 112-g pump bottle that delivers 20 mg of diclofenac per 1 g of solution per actuation.14 Patients are to apply 2 actuations (40 mg) either into the hand and then onto the knee or directly to the knee twice daily. If pain is located in both knees, two pumps are to be used on each knee twice daily. The solution is then to be spread to all areas of the knee (front, back, and sides). Prior to first use, the pump needs to be primed by depressing it four times fully and discarding the pumped solution.14
The appearance of the diclofenac topical solution ranges from colorless to slightly pink or orange, and the bottle should be stored at room temperature.12-14 It is important to inform patients that the skin must be intact and healthy prior to application to avoid potential overdosage. Hand washing should commence after application. This product differs slightly from the others in that clothing should not be applied until the area is completely dry. Use with heat or occlusive dressing and during exercise has not been studied and is thus not recommended.12-14
The most commonly experienced adverse events for topical diclofenac include pruritus, dry skin, and contact dermatitis.8 The risk of adverse GI effects and renal failure is shown to be significantly decreased with topical administration of diclofenac compared to systemic administration.8 Chronic use of NSAIDs can cause hypertension, so caution and monitoring should occur in patients with preexisting hypertension.12-14
LIDOCAINE
Pain is transmitted along the afferent nerve fibers to the central nervous system.8 Interruption of this transmission by local anesthetics such as lidocaine occurs via the reversible inhibition of the sodium-potassium channels.8 Lidocaine is available in many formulations. For chronic peripheral neuropathic pain relief, the patch is most commonly utilized.7
Lidocaine 5% Patch
The lidocaine 5% patch is available in a carton of 30 individually wrapped patches, each containing 700 mg of lidocaine.15 These patches should be stored at room temperature. They are to be applied once a day to intact skin on the most painful site. Removal after 12 hours is necessary to prevent elevated blood concentrations of lidocaine, which can lead to serious adverse events such as cardiac dysrhythmia and methemoglobinemia.16 Up to three patches may be applied at once, and they may be cut to smaller sizes.15 While clothing may be worn over the patch, occlusive dressings and heat can increase absorption of the anesthetic and should be avoided. Contact with water should be avoided due to negative effects on the adhesive. After handling of the patch, hand washing should take place, and upon removal the patch should be folded adhesive sides together.15
The dosing for these topical diclofenac and lidocaine formulations is summarized in TABLE 2.10-12,14,15
SPECIAL POPULATIONS
Pediatrics
The World Health Organization also suggests that the Faces Pain Scale-Revised is suitable for rating of pain in the pediatric patient.17 At this time, the safety and efficacy of neither the topical diclofenac solution, patch, or gel nor the lidocaine patch have been established in children.10-15 The use of these products in the pediatric population is not currently recommended.10-15
Geriatrics
Chronic pain is associated with increased falls, debility, depression, anxiety, sleep and appetite impairment, and a poorer quality of life in the geriatric population.18,19 Due to the physiological changes that occur with normal aging, pharmacokinetic and pharmacodynamic changes occur with medication use in the elderly.19,20 Importantly, this increases the risk for adverse events, such as the confusion and sedation commonly seen with medications for pain management.19,20 Current guidelines recommend that pharmacologic management of chronic pain use the least invasive method possible.19,20 In clinical trials with diclofenac solution and gel, the incidence of adverse events was not significant when comparing geriatric and nongeriatric patients.10,12-14 There were not enough geriatric patients included in the diclofenac patch trials to establish whether a different response occurred.11 There are no recommendations for dosage adjustment in the geriatric population for the topical lidocaine 5% patch.15,16
Pregnancy and Lactation
The diclofenac solution and patch are designated Pregnancy Category C prior to 30 weeks gestation; after this point they have been assigned a Category D due to the increase potential for premature closure of the ductus arteriosus in the fetus.11-14 While the diclofenac gel has only been assigned Category C, the manufacturer states that the product should be avoided in late pregnancy.10 The lidocaine 5% patch is currently labeled as Pregnancy Category B.15 Rate of excretion into the breast milk of these products is unknown; thus, none of these products is recommended for use in lactation.10-15
NONPHARMACOLOGIC MANAGEMENT
Nonpharmacologic pain management strategies that have shown efficacy in clinical trials include cognitive behavioral therapy (CBT), exercise, heat therapy, rehabilitation, spinal manipulation, acupuncture, massage, self-management education programs, tai chi, and yoga.6,8,21-23 Heat and cold therapy has been a mainstay in the management of soft-tissue injury.8 While heat therapy has supportive evidence for pain relief, cold therapy does not.8,22 Cold therapy is thought to reduce pain by causing vasoconstriction, thus reducing inflammation, while heat therapy is proposed to decrease muscle stiffness.8 Nonpharmacologic management should accompany pharmacologic management when possible.6,21
CONCLUSION
Management of chronic pain is complicated and many of the available medication options can lead to unwanted adverse effects. Topical prescription nonopioid therapies (lidocaine and diclofenac) are a good option for management of select states of chronic pain. As with all medication therapy, care must be taken to ensure that the drug is appropriate for the patient. It is important for pharmacists to be aware of the available options in order to contribute to the care of those who have chronic pain.
REFERENCES
1. National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention. Prescription painkiller overdoses. CDC. Updated July 2013. www.cdc.gov/vitalsigns/PrescriptionPainkillerOverdoses/index.html. Accessed December 20, 2014.
2. Schedules of controlled substances: rescheduling of hydrocodone combination products from schedule III to schedule II (Docket No. DEA-389). Fed Regist. 2014;163(79):11037-11045.
3. Cohen S, Raja S. Chapter 29: Pain. In: Goldman L, Schafer A, eds. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Saunders/Elsevier; 2012:133.
4. The American Academy of Pain Medicine. AAPM facts and figures on pain. www.painmed.org/PatientCenter/Facts_on_Pain.aspx. Accessed December 20, 2014.
5. Stanos SP, Galluzzi KE. Topical therapies in the management of chronic pain. Postgrad Med. 2013;125(4 suppl 1):25-33.
6. Baumann T, Herndon CM, Strickland J. Chapter 44: Pain management. In: DiPiro J, Talbert RL, Yee G, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014.
7. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251.
8. Barkin RL. The pharmacology of topical analgesics. Postgrad Med. 2013;125(4 suppl 1):7-18.
9. McPherson ML, Cimino NM. Topical NSAID formulations. Pain Med. 2013;14(suppl 1):S35-S39.
10. Voltaren gel (diclofenac sodium 1%) package insert. Parsippany, NJ: Novartis; 2009.
11. Flector patch (diclofenac epolamine 1.3%) package insert. Bristol, TN: King Pharmaceuticals; August 2011.
12. Pennsaid 1.5% (diclofenac sodium topical solution) package insert. Hazelwood, MO: Mallinckrodt Brand Pharmaceuticals, Inc; October 2013.
13. Diclofenac sodium 1.5% package insert. Minneapolis, MN: Perrigo; July 2014.
14. Pennsaid 2% (diclofenac sodium topical solution ) package insert. Hazelwood, MO: Mallinckrodt Brand Pharmaceuticals, Inc; August 2014
15. Lidoderm (lidocaine 5% patch) package insert. Malvern, PA: Endo Pharmaceuticals; July 2014.
16. Lidoderm. In: DRUGDEX System. Greenwood Village, CO: Thomson Healthcare. Updated periodically. www.thomsonhc.com/micromedex. Accessed December 20, 2014.
17. World Health Organization. WHO Guidelines on the Pharmacological Treatment of Persisting Pain in Children With Medical Illnesses. Geneva, Switzerland: WHO; 2012. http://whqlibdoc.who.int/publications/2012/9789241548120_Guidelines.pdf. Accessed December 20, 2014.
18. Makris UE, Abrams RC, Gurland B, Reid MC. Management of persistent pain in the older patient: a clinical review. JAMA. 2014;312(8):825-836.
19. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.20. Pergolizzi J, Boger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8(4):287-313.
21. Makris UE, Abrams RC, Gurland B, Reid MC. Management of persistent pain in the older patient: a clinical review. JAMA. 2014;312(8):825-836.
22. Chou R, Huffman LH, American Pain Society, American College of Physicians. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College Of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):492-504.
23. Simpson EL, Duenas A, Holmes MW, et al. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation. Health Technol Assess. 2009;13(17):iii, ix-x, 1-154.
To comment on this article, contact rdavidson@uspharmacist.com.





