US Pharm. 2007;32(9):45-53.
Rosacea
is a common, chronic skin disorder characterized by transient or persistent
central facial erythema, telangiectasia (visible blood vessels), inflammatory
episodes with papules and pustules, and, in severe cases, rhinophyma.
1,2 It is estimated that approximately 14 million people in the United
States are diagnosed with some form of this dermatosis. Rosacea most
frequently occurs in people between the ages of 30 and 50 years, with women at
two to three times greater risk than men. Northern European descendants are at
greatest risk for developing this condition.2
The National Rosacea Society
Expert Committee developed a standard classification system based on the
morphologic characteristics of the condition. The system identifies the
primary and secondary signs and symptoms of rosacea.3 Primary signs
and symptoms include transient erythema, nontransient erythema, papules,
pustules, and telangiectases.3 If one or more of the primary signs
concomitantly occur with central face distribution, rosacea is suspected.
Patients who are diagnosed with rosacea and exhibit one or more of the primary
features often present with the secondary signs and symptoms. These include
burning or stinging, plaques, dry appearance, edema, ocular manifestations,
and phymatous changes. Secondary features usually appear in the presence of
the primary symptoms but can also occur in the absence of primary signs and
symptoms.3 Due to the various and numerous manifestations of the
disease, the National Rosacea Society Expert Committee has created subtypes of
the condition.
Classification
There are four
major subtypes and several other nonclassic subtypes that describe the most
common patterns associated with the signs and symptoms of rosacea. The
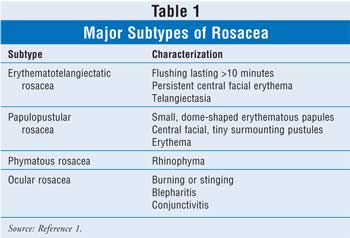
subtypes include erythematotelangiectatic rosacea (ETR), papulopustular
rosacea (PPR), phymatous rosacea, and ocular rosacea (TABLE 1).1
ETR is the most common subtype and is characterized by flushing, which
usually lasts longer than 10 minutes, accompanied by persistent central facial
erythema. Telangiectasia is usually prominent on the cheeks and nose in
patients with the ETR subtype and may contribute to erythema. This subtype of
rosacea responds poorly to treatment.1 PPR typically presents as
small, dome-shaped erythematous papules with tiny surmounting pustules on the
central portion of the face. This subtype, which is fairly uncommon, is also
associated with erythema and telangiectatic vessels.1 The most
frequent manifestation of this subtype is rhinophyma. Rhinophyma can be a
disfiguring condition of the nose that results from hyperplasia of both the
sebaceous glands and the connective tissue. Although rosacea is more frequent
among women, rhinophyma is predominant among men, with the ratio being
approximately 20:1.1 The last subtype, ocular rosacea, is common
but often misdiagnosed. This type of rosacea can manifest as blepharitis and
conjunctivitis with inflamed eyelids and meibomian glands; however, most
patients present with mild symptoms, such as burning or stinging in the eye.
This may precede, follow, or occur simultaneously with other classic rosacea
symptoms.1
Other nonclassic subtypes of
rosacea include glandular rosacea and granulomatous rosacea. Glandular rosacea
is characterized by thick, sebaceous skin; papules and pustules; and
nodulocystic lesions. Granulomatous rosacea is described as the presence of
yellow, brown, or red papules or lesions on the cheeks or around the mouth and
eyes. Granulomatous rosacea may be classified as granulomatous facial
dermatitis rather than a type of rosacea, because these patients do not have
persistent erythema and usually present with lesions outside the central face
or with disease in a unilateral distribution. Such patients are less likely to
have the flushing, burning, and stinging characteristic of other subtypes.
1
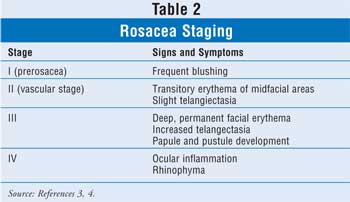
The development of rosacea
occurs in four stages (TABLE 2). Stage I is described as prerosacea. In
this stage, rosacea-induced blushing is the main symptom and can develop as
early as childhood. Stage II is mainly vascular; the disease progresses into
transitory erythema of the midfacial area, and mild telangiectasia begins to
develop. During stage III, facial redness worsens, becoming deeper and
permanent. Also during this stage, telangiectasia increases, ocular changes
begin to develop, and papule and pustule formation occurs. In stage IV, there
is continued and increased skin and ocular inflammation. The ocular
inflammation can ultimately result in visual loss. It is also in this stage
that fibroplasia and sebaceous hyperplasia of the skin lead to rhinophyma.
4
Etiology
The exact etiology
of rosacea remains unknown; however, several factors have been implicated in
its pathogenesis.1-2,4 The erythema is caused by dilation of the
superfacial vasculature of the face. This increased blood flow to the
superfacial vasculature results in edema. It has been proposed that
Helicobacter pylori may be a cause of this disease.4 Recent
studies have also linked H pylori with urticaria, Schonlein-Henoch
purpura, and Sjogren's syndrome. It remains controversial whether there is
benefit from the eradication of H pylori on the symptoms associated
with rosacea.4 Other possible etiologies include climatic exposure,
ingested chemical agents, abnormalities in cutaneous vascular homeostasis,
endothelial damage and matrix degeneration of the skin, and microbial
organisms (e.g., Demodex folliculorum).1,4
Prior to initiating treatment,
factors that trigger signs and symptoms should be identified and, if possible,
avoided. Triggers are patient-specific; however, the most common known
triggers include hot or cold temperatures, wind, hot drinks, exercise, spicy
food, alcohol, emotional stress, topical products, menopausal flushing, and
medications that may induce flushing (i.e., niacin, disulfiram, nitroglycerin).
5,6 Some important preventive measures that patients with rosacea can
take include the daily use of a gentle sunscreen, avoidance of midday sun, and
the use of protective clothing.5,6 Alcohol consumption is not known
to be a direct cause of the disease, but it can aggravate the condition
through peripheral vasodilation.5 Only hypoallergenic and
nonirritating facial cleansers, lotions, and cosmetics should be used in these
patients.4,5
Cure Still Elusive
The cure for
rosacea remains elusive, and all of the medications currently used will only
help in resolving symptoms but will not completely eradicate the disease.
Therefore, the goal in managing rosacea is to control the symptoms as opposed
to eradicating the disease. Rosacea should be treated within the
early stages to prevent progression to edema and irreversible fibrosis.
Treatment typically depends on the subtype and stage of rosacea.1,4
Both topical and oral medications are used in the treatment of rosacea, and
those agents most commonly prescribed include topical metronidazole, sodium
sulfacetamide–sulfur cleanser, azelaic acid, and oral tetracycline and
macrolide antibiotics. When treating rosacea, therapy should be initiated with
a combination of both oral and topical products, as this regimen has been
shown to reduce the initial prominent symptoms, prevent relapse when oral
therapy is discontinued, and maintain long-term control. Oral therapy is
usually continued until inflammation dissipates or for a maximum of 12
weeks--whichever comes first. Antibiotics have traditionally been considered
first-line therapy, primarily due to their anti-inflammatory effects as
opposed to their antimicrobial action alone.4 The tetracyclines and
macrolides are the most commonly prescribed antibiotics for the treatment of
rosacea.
Topical Treatments
To date, there are
only three FDA-approved topical medications for the treatment of rosacea,
particularly for the management of papules, pustules, and erythema. The three
approved topical medications include 0.75% and 1% metronidazole, 10% sodium
sulfacetamide with 5% sulfur, and 15% azelaic acid gel. Other medications that
are not FDA approved for the treatment of rosacea but have shown some
beneficial effects include benzoyl peroxide, clindamycin, retinoids, and
topical steroids.5,6
Several randomized, double-blind,
placebo-controlled trials involving the use of topical metronidazole have
indicated that it is safe and effective in the treatment of rosacea.7-12
The pharmacologic mechanism responsible for the effectiveness of
metronidazole in the treatment of rosacea remains unclear; however, in
vitro studies have shown that metronidazole interferes with the release of
reactive oxygen species from neutrophils that cause tissue injury at the site
of inflammation.13 Clinical trials suggest that topical
metronidazole is most effective at reducing inflammatory lesions and erythema
associated with rosacea.14 In addition, one of the other benefits
to using topical metronidazole is the lack of systemic toxicity that is noted
with the oral formulation.13 Although it is generally used as
first-line treatment and continues to be one of the most widely prescribed
medications in the treatment of rosacea, the optimal dose of metronidazole for
this condition has yet to be determined. Daily dosing and twice-daily dosing
of the 1% and 0.75% formulations, respectively, have proven to be effective
for this condition. Currently, topical metronidazole is available as a
twice-daily application of 0.75% cream or gel and as a once-daily 1% cream.
The 0.75% formulation was originally approved as a twice-daily application
based on its half-life of six hours. However, recent data suggest that
metronidazole is metabolized into active metabolites that may prolong its
efficacy, and once-daily dosing of the 0.75% cream is now regarded as an
acceptable form of treatment.5,13 The effectiveness of the
once-daily 0.75% formulation was found to be equivalent to the once-daily 1%
formulation in a 12-week, randomized trial that included 72 patients. No
significant difference existed between the treatment groups with regard to
reduction of erythema, papules, and pustules; treatment failure; dryness;
safety; and global assessment of severity.12
Topical metronidazole has
shown to reach significant reduction of erythema as early as week 2 and as
late as week 10 depending on the formulation.10,11 In a
double-blind, randomized clinical trial, the 1% formulation significantly
reduced inflammatory lesions by week 4.10 Maintenance therapy is a
critical aspect of rosacea therapy. Typically, after discontinuing treatment,
relapse occurs in one fourth of patients after one month and in two thirds of
patients after six months.14 In a randomized, double-blind,
placebo-controlled clinical trial, topical metronidazole effectively
maintained remission over six months in those patients who were previously
treated with combination therapy including tetracycline and topical
metronidazole.15 After discontinuing oral medication in a
multicenter, randomized, double-blind trial, topical metronidazole maintained
remission longer than placebo, and 23% of patients relapsed with metronidazole
gel compared to 42% with the placebo cream.15,16 Topical
metronidazole is poorly absorbed with either undetectable or trace serum
concentrations reported after its use.13 Topical metronidazole is
generally well tolerated, with adverse events reported in less than 5% of
patients. The most common reactions reported were local reactions including
dryness, redness, pruritis, burning, and stinging.13
The combination of sodium
sulfacetamide 10% and sulfur 5% provides a safe, well-tolerated, and effective
option for the treatment of rosacea that may be less irritating than
metronidazole.4,5 Traditionally, this combination's use was limited
because of its unpleasant odor; however, it is now formulated as a cleanser
with a masked odor. This new formulation has led to the reemergence of this
product.6 The proposed mechanism of action of these agents in the
treatment of rosacea is attributed to the antibacterial properties of
sulfacetamide and its ability to compete with para-aminobenzoic acid along
with the keratolytic properties of sulfur, which is believed to have an
anti-inflammatory effect.17 In an eight-week, double-blind,
placebo-controlled trial, the combination of sulfacetamide and sulfur
decreased inflammatory lesions by 78%, compared to a 36% decrease in the
placebo arm. The sodium sulfacetamide–sulfur combination also decreased
erythema by 83%, compared to a 31% decrease in the placebo group.18
This twice-a-day cleanser is effective as monotherapy and has been shown to
significantly reduce papule count and erythema.19 However, the use
of sodium sulfacetamide-sulfur cleanser followed by metronidazole cream has
proven to be superior to the cleanser alone in reducing papule counts and
overall rosacea severity.19 The newer wash on/wash off sodium
sulfacetamide–sulfur formulation has lower irritation potential, improved
absorption through hydrated skin, less lingering odor, and fewer drug–drug
interactions with other topical regimens or cosmetics.5 Most of the
adverse reactions associated with sodium sulfacetamide-sulfur use are mild and
include pruritis, contact dermatitis, irritation, and xerosis. The combination
of sodium sulfacetamide and sulfur is contraindicated in patients with a
sulfonamide hypersensitivity.20
Azelaic acid 15% gel is the
most recently FDA-approved topical agent for the treatment of rosacea. It is a
naturally occurring saturated dicarboxylic acid similar to metronidazole and
is thought to inhibit the reactive oxygen species produced by neutrophils.
5 The efficacy and safety of azelaic acid gel in PPR was investigated in
two vehicle-controlled, randomized, phase III trials involving 664 subjects.
21 Improvement of erythema occurred in 44% and 46% of patients in the
azelaic acid groups compared to 29% and 28% improvement in the placebo-treated
patients. From baseline, the mean reductions of inflammatory lesions were 58%
and 51% in the azelaic acid–treated groups compared to 40% and 39% in the
control groups. Burning, stinging, or itching was experienced by 38% of the
patients treated with azelaic acid. Scaling, dry skin, and rash occurred in
approximately 12% of the azelaic acid–treated patients. The majority of these
side effects were transient in those affected.21 In a smaller
randomized, double-blind, parallel trial, azelaic acid 15% was compared to
metronidazole gel 0.75% in patients with PPR. Patients received either azelaic
acid or metronidazole twice daily for 15 weeks. Azelaic acid gel was
significantly more effective at reducing inflammatory lesions and the mean
lesion count.22 There was a 72.7% decrease in the inflammatory
lesions in the azelaic acid group compared to a 55.8% decrease in the
metronidazole group. Azelaic acid also significantly improved erythema
severity, as 56% of the patients treated with azelaic acid improved versus 42%
of metronidazole-treated patients.22 There were no serious or
systemic adverse effects reported in either treatment group. However, 26% of
the azelaic acid treatment group experienced facial skin reactions and
symptoms compared to 7% in the metronidazole-treated patients.22
Benzoyl peroxide,
erythromycin, and clindamycin have all been used as topical agents for the
treatment of rosacea; however, none of them has been FDA approved because
there is limited data available to support the use of these topical products
for this disorder; they are only to be used as alternative therapies.5
Benzoyl peroxide is typically used in patients with phymatous and glandular
rosacea. The use of erythromycin for rosacea was prompted by its successful
treatment of acne vulgaris. This twice-daily topical preparation has been
shown to reduce erythema and suppress papules and pustules after four weeks of
treatment.23 Topical clindamycin is primarily used in the treatment
of acne, but it can be an effective alternative to tetracycline and topical
metronidazole in patients with rosacea who are pregnant.5,24,25
Oral Antibiotics
Oral antibiotics
have been a mainstay of rosacea therapy for more than 40 years. They have
proven to be effective in reducing the signs and symptoms associated with this
condition. Historically, rosacea was thought to be a result of a bacterial
infection, and since the 1950s oral antibiotics have been used as off-label
therapy.26 Currently, there is only one FDA-approved antibiotic for
the treatment of rosacea, the tetracycline doxycycline (Oracea). The number of
FDA-approved antibiotics for the treatment of rosacea is limited, primarily
because compelling evidence that the disorder is secondary to a bacterial
infection is lacking. Tetracycline and its derivatives minocycline and
doxycycline are the principal oral antibiotics of choice for the treatment of
rosacea.26 The tetracyclines have the ability to down-regulate
production of proinflammatory cytokines such as interleukin-1 and tumor
necrosis factor alpha. They also inhibit proteolytic enzymes produced by
inflammatory cells that degrade collagen, thereby decreasing inflammation that
is seen during the inflammatory response in rosacea.26
Tetracyclines are most effective against PPR; however, relapse rates are high
if they are used as monotherapy without a topical agent.24,26
Traditionally, tetracycline 250 to 1,000 mg daily and doxycycline or
minocycline 100 to 200 mg daily for three to four weeks have been the most
common dosages used to achieve substantial improvement in the signs and
symptoms.26
In May 2006, the FDA approved
doxycycline (Oracea) 40 mg once daily, the first oral medication approved for
the treatment of rosacea. It is only indicated for the treatment of
inflammatory lesions (papules and pustules) in adult patients.28 To
date, there have been two phase III clinical trials involving doxycycline.
Both of these studies were double-blind, placebo-controlled trials and were
conducted simultaneously. A total of 537 patients received either placebo or
doxycycline 40 mg once daily for 16 weeks. In both studies, doxycycline
significantly reduced inflammatory lesions compared to placebo. In the two
studies, the doxycycline-treated patients had a mean reduction in inflammatory
lesion count of 61% and 46% compared with 29% and 20% in those who received
placebo.29 There are also data supporting the use of doxycycline 20
mg twice daily in those patients with inflammatory lesions and erythema.
5,27 The twice-a-day regimen is believed to have fewer adverse reactions
and is less likely to cause bacterial resistance because it results in
subantimicrobial blood levels.26 Minocycline and doxycycline have
longer elimination half-lives and improved bioavailability compared to the
parent compound, which can prolong their duration of action and minimize
gastrointestinal (GI) side effects.5 Possible adverse reactions
that may occur with the use of tetracycline include GI irritation, rash, renal
toxicity, hepatic cholestasis, anemia, thrombocytopenia, and hypersensitivity
reactions. The tetracyclines are contraindicated in pregnancy and in children
younger than 8 years.30
The oral macrolides
erythromycin, clarithromycin, and azithromycin have been used for the
treatment of PPR.26 The macrolides prevent bacterial growth by
interfering with protein synthesis. They inhibit the translocation of peptides
by binding to 50S of the bacterial ribosome. These antibiotics are most
commonly used when intolerance, pregnancy, resistance, or allergies prevent
the use of tetracyclines.5 One advantage of the use of the
second-generation macrolides clarithromycin and azithromycin over erythromycin
is that they have a faster onset of action and less GI irritation than
erythromycin.31,32 Clarithromycin 250 to 500 mg twice daily for six
weeks has been found to be as effective as doxycycline with a more tolerable
side-effect profile.31 In a trial that compared clarithromycin 250
mg twice daily for four weeks followed by clarithromycin 250 mg once daily for
four weeks to doxycycline 100 mg twice daily for four weeks followed by
doxycycline 100 mg daily for four weeks, clarithromycin reduced erythema and
papules at a faster rate. The authors concluded that six weeks of
clarithromycin treatment was as efficacious as eight weeks of doxycline
treatment.31 Azithromycin 250 mg for 12 weeks proved to decrease
inflammatory lesions by 89% from baseline.32 Clarithromycin and
azithromycin are preferred over erythromycin because of better tolerance and
improved bioavailability; however, these second-generation macrolides may be
more expensive.26 Larger, controlled clinical trials are necessary
to determine the exact role of the second-generation macrolides in both
initial and maintenance therapy for rosacea.
Oral metronidazole may serve
as another alternative for those who cannot tolerate tetracyclines or for
those who have been treated unsuccessfully with tetracycline. A double-blind,
randomized trial evaluated the efficacy of oral metronidazole in rosacea
treatment. Patients received oral metronidazole 200 mg twice daily or
oxytetracyline 250 mg twice daily. Both therapies showed sustained improvement
at 12 weeks.5
In some patients, isotretinoin
can be used for refractory rosacea, as it reduces the size of sebaceous glands
and alters keratinization. Small clinical trials have demonstrated that
isotretinoin may reduce the number of papules and pustules, erythema, and
nasal volume in rhinophyma in patients with refractory rosacea.5
More recently, Erdogan et al. evaluated low-dose isotretinoin 10 mg daily for
four months in patients with treatment-resistant rosacea.33
Isotretinoin significantly reduced inflammatory lesions, erythema, and
telangiectasia. Use of isotretinoin is often limited due to its serious and
abundant side-effect profile. The most common adverse effects include bone or
joint pain, burning, redness, itching, eye inflammation, nosebleeds,
scaling, skin infection, and rash. Isotretinoin is contraindicated in
pregnancy, as it is teratogenic; for this reason, it has to be prescribed
under a special restricted distribution program.34 Information
regarding isotretinoin's optimal dosage and duration of therapy for the
treatment of rosacea is limited. In addition, the majority of the clinical
trials evaluating its safety and efficacy involve small sample sizes.
Therefore, more studies involving larger patient populations are needed to
determine the optimal dosage and duration of therapy.
Laser and Light Therapies
Vascular laser therapy and light
therapy serve as additional options for the treatment of telangiectasia in
patients who do not respond to conventional therapy. Laser and light therapy
have the capability to reorganize and remodel dystrophic dermal connective
tissue and strengthen the epidermal barrier by thermally inducing fibroblasts
and endothelial proliferation or by causing endothelial disruption, leading to
cytokine, growth factor, and heat shock protein activation.35 The
vascular laser therapies currently used for telangiectasia and erythema are
the standard pulse-dye laser (585 or 595 nm), long pulsed-dye lasers (595 nm),
the potassium titanyl phosphate laser (532 nm), and the diode-pumped
frequency-doubled laser (532 nm). The short-wavelength lasers (541 and 577 nm)
induce vessel destruction without causing collateral tissue damage.35
Therefore, the short-wavelength vascular lasers are preferred for superficial
red vessels and persistent erythema. Intense pulsed-light therapy penetrates
the skin deeper than vascular laser therapy and is best suited for vascular
lesions and pigmented lesions. Its main benefits are the ability to treat
larger and deeper vessels and promote collagen remodeling. Laser and light
therapies may require one to three treatments four to eight weeks apart to
achieve the best results; however, their use is limited due to cost.35,36
Summary and Conclusion
Currently, rosacea
treatment is aimed at reducing symptoms and improving facial appearance. Many
questions still remain regarding the pathogenesis and etiology of the disease.
Even though a definitive cause has not been determined, therapy should begin
with avoiding possible triggers. If a patient is still experiencing rosacea
symptoms once triggers have been identified and, if possible, removed, topical
metronidazole remains the first-line therapy for the treatment of rosacea.
Other topical agents such as sodium sulfacetamide–sulfur combination, azelaic
acid, benzoyl peroxide, erythromycin, and clindamycin can be used as an
alternative to metronidazole. Oral antibiotics, which can prevent relapse and
maintain remission, are often used in combination with the topical agents.
Recently, the first oral antibiotic, doxycycline, was FDA approved for the
treatment of inflammatory lesions associated with rosacea. If topical and oral
treatments are unsuccessful, vascular laser and light therapies are options
for refractory cases.
Even though there are a
variety of therapeutic options for the treatment of rosacea, investigation of
genetic factors and the histologic and pathologic basis of papules and
pustules still need to be carried out; this will lead to new and improved
treatment options and may help decrease psychosocial distress of affected
individuals. Despite the lack of understanding, therapy has improved since the
diagnostic criteria have become more uniform.
References
1. Crawford G,
Pelle M, James W. Rosacea: I. Etiology, pathogenesis, and subtype
classification. J Am Acad Dermatol. 2004;51:327-341.
2. Fernandez A. Oral
use of azithromycin for the treatment of rosacea. Arch Dermatol.
2004;140:489-490.
3. Wilkin J, Dahl M,
Detmar M, et al. Standard grading system for rosacea: Report of the national
rosacea society expert committee on the classification and staging of rosacea.
J Am Acad Dermatol. 2004;50:907-912.
4. Cohen A, Tiemstra J.
Diagnosis and treatment of rosacea. J Am Board Fam Pract.
2002;15:214-217.
5. Pelle M, Crawford G,
James W. Rosacea: II. Therapy. J Am Acad Dermatol. 2004;51:499-512.
6. Nally J, Berson D.
Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-27.
7. Nielsen PG. A double
blind study of 1% metronidazole cream versus systemic oxytetracycline therapy
for rosacea. Br J Dermatol. 1983;109:63-65.
8. Nielsen PG.
Treatment of rosacea with 1% metronidazole cream. A double blind study. Br
J Dermatol. 1983;108:327-332.
9. Bleicher PA, Charles
JH, Sober AJ. Topical metronidazole therapy for rosacea. Arch Dermatol.
1987;123:609-614.
10. Breneman D, Stewart
D, Hevia O, et al. A double-blind, multicenter clinical trial comparing
efficacy of once daily metronidazole 1% to vehicle in patients with rosacea.
Cutis. 1998;61:44-47.
11. Jorizzo J, Lebwohl
M, Tobey R. The efficacy of metranidazole 1% cream once daily compared with
metronidazole 1% twice daily and their vehicles in rosacea: a double blind
clinical trial. J Am Acad Dermatol. 1998;39:502-504.
12. Dahl M, Jarratt M,
Kaplan D, et al. Once daily topical metronidazole cream formulations in the
treatment of papules and pustules of rosacea. J Am Acad Dermatol.
2001;45:723-730.
13. Zip C. An update on
the role of topical metronidazole in rosacea. Skin Therapy Lett.
2006;11:1-4.
14. Del Rosso J.
Topical therapy for rosacea: a status report. Practical Dermatology.
2004:43-46.
15. Dahl MV, Katz HL,
Krueger GG, et al. Topical metronidazole maintains remission of rosacea.
Arch Dermatol. 1998;134:679-683.
16. Wilkin J. Use of
topical products for maintaining remission in rosacea. Arch Dermatol.
1999;135:79-80.
17. Mackley CL,
Thiboutot DM. Diagnosing and managing the patient with rosacea. Cutis.
2005;75:25-29.
18. Saunder D, Miller
R, Gratton D, et al. The treatment of rosacea: safety and efficacy of sodium
sulfacetamide 10% and sulfur 5% lotion is demonstrated in a double blind
study. J Dermatol Treat. 1997;8:79-85.
19. Del Rosso J. A
status report on the medical management of rosacea: focus on topical
therapies. Cutis. 2002;70:271-275.
20. Lacy C, Armstrong
L, Goldman M, et al. Drug Information Handbook. 10th ed. Hudson, OH:
Lexi-Comp; 2002-2003:1268-1269.
21. Thiboutot D,
Theiroff R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as new
treatment for papulopustular rosacea: results from two randomized phase III
studies. J Am Acad Dermatol. 2003;48:836-845.
22. Elewski B,
Fleischer A, Pariser D. A comparison of 15% azelaic acid gel and 0.75%
metronidazole gel in the topical treatment of papulopustular rosacea. Arch
Dermatol. 2003;139:1444-1450.
23. Mills H, Kligman M.
Topically applied erythromycin in rosacea. Arch Dermatol.
1976;112:553-554.
24. Blount W, Pelletier
A. Rosacea: a common, yet commonly overlooked, condition. Am Family
Physician. 2002;66:435-442.
25. Wilkin J, Dewitt S.
Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J
Dermatol. 1993;32:65-67.
26. Bladwin H. Oral
therapy for rosacea. J Drugs Dermatol. 2006;5:16-21.
27. Bikowski J.
Subantimicrobial dose doxycycline for acne and rosacea. SKINmed.
2003;2:234-245.
28. Oracea
[package insert]. Newton, PA: CollaGenex Pharmaceuticals Inc.; May 2006.
29. Rosso D. Results of
phase 3 clinical trials of Oracea. Dermatology Times. 2005;26(9):34.
Abstract.
30. Lacy C, Armstrong
L, Goldman M, et al. Drug Information Handbook. 10th ed.
Hudson, OH: Lexi-Comp; 2002-2003:1306-1308.
31. Torresani C, Pavesi
A, Manara G. Clarithromycin versus doxycycline in the treatment of rosacea.
Int J Dermatol. 1997;36:942-946.
32. Bakar O, Demircay
Z, Gurbuz O. Therapeutic potential of azithromycin in rosacea. Int J
Dermatol. 2004;43:151.
33. Erdogan F,
Yurtsever P, Aksoy D, et al. Efficacy of low dose isotretinoin in patients
with treatment-resistant rosacea. Arch Dermatol. 1998;134:884-885.
34. Accutane [package
insert]. Nutley, NJ: Roche Inc.; August 2005.
35. Lonne-Rahm S,
Nordlind K, Wiegleb D, et al. Laser Treatment of Rosacea. Arch Dermatol
. 2004;140:1345-1349.
36. Sadick N. A
structural approach to nonablative rejuvenation. Cosmetic Dermatol.
2002;15:39-43.
To comment on this article, contact uspharmacist.com.





