ABSTRACT: Bacterial meningitis is a serious infection that requires immediate treatment. Recommended empiric antimicrobial therapy is based upon the most likely pathogen, according to a patient’s age and immune status. Antimicrobial therapy should be modified after identification of the causative microorganism and results of susceptibility tests. Preventive measures include the use of vaccines that target Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae, as well as the use of chemoprophylaxis in selected situations. Pharmacists are in a key position to recommend appropriate antimicrobial therapy for the treatment and prophylaxis of bacterial meningitis and to ensure that patients are receiving recommended vaccinations.
Meningitis is an inflammation of the meninges, which are the membranes that cover the brain and spinal cord.1 Meningitis may have a noninfectious or infectious etiology.2,3 Bacterial meningitis, which occurs after a bacterial pathogen invades the cerebrospinal fluid (CSF) of the subarachnoid space, is considered to be one of the most severe forms of infection.2 Bacterial meningitis may be acquired in the community, or it may be a healthcare-associated infection related to an invasive procedure or head trauma.2,4 This article reviews the management of community-acquired bacterial meningitis and the vaccines and medications available for prevention of infection.
Etiology
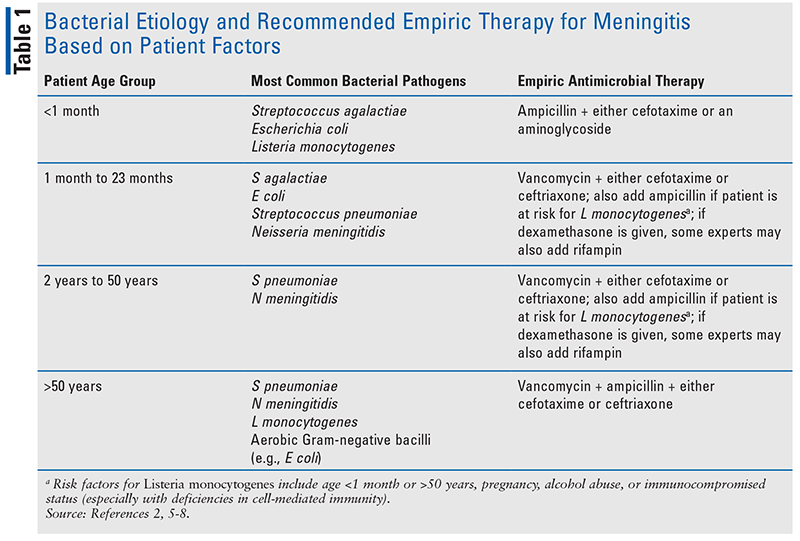
The most common bacterial etiology of community-acquired meningitis varies based on age and immune status as indicated in Table 1.2,5-8 Also, Haemophilus influenzae type b may be a pathogen in the young, elderly, or those with recurrent infection, although this bacterium is not a common cause of meningitis in the United States because this vaccine is part of routine recommended childhood immunizations.1,8,9
Clinical Presentation and Diagnosis
The most common symptoms of bacterial meningitis are headache, fever, neck stiffness, and/or altered mental status, with 95% of patients presenting with at least two of these symptoms.2,10 Although bacterial meningitis has been associated with a “classic triad” of fever, neck stiffness, and altered mental status, one study found that this triad of symptoms was reported in fewer than half of adult patients.2,10 Additional symptoms of bacterial meningitis include focal neurological deficits, nausea/vomiting, or petechial rash, with petechial rash associated with infection caused by Neisseria meningitidis.5 Infants and children often have nonspecific symptoms such as irritability, poor feeding, vomiting, or seizures.5
Blood cultures and a lumbar puncture (LP) to collect CSF fluid for analysis should be performed upon suspicion of bacterial meningitis.6 In certain situations, a CT scan of the head is required prior to LP to exclude a mass lesion or another cause of increased intracranial pressure, which may lead to brain herniation from the procedure.6 In bacterial meningitis, CSF findings typically include an elevated white blood cell count (usually >1,000 cells/mm3, although this can vary), with more than 80% neutrophils; elevated protein; low glucose; and a low CSF-to-serum glucose ratio.5,6 Isolation of bacteria from the CSF or blood in a patient with CSF findings consistent with bacterial meningitis confirms the diagnosis, and results of testing should be used to guide antimicrobial treatment.2,6
TREATMENT
Empiric Treatment
If the LP is delayed for any reason—including the need for additional diagnostic testing, such as a CT scan of the head—empiric antimicrobial therapy should be started as soon as possible, ideally after blood cultures have been performed.6 It is important to start antimicrobial therapy even if the evaluation for bacterial meningitis is ongoing, since a delay in treatment is associated with increased morbidity and mortality.6 Recommended empiric treatment of bacterial meningitis is based on a patient’s age and comorbid conditions (Table 1).2,6 The empiric antimicrobial regimen should include ampicillin for those who are at risk for infection from Listeria monocytogenes.2,6,11 For those with a severe penicillin allergy, trimethoprim/sulfamethoxazole or meropenem can be used as an alternative agent for coverage of this pathogen.2,6,11
Directed Treatment of Selected Pathogens
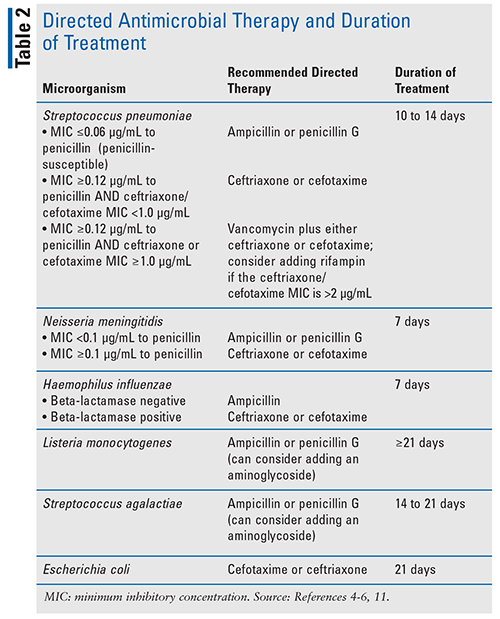
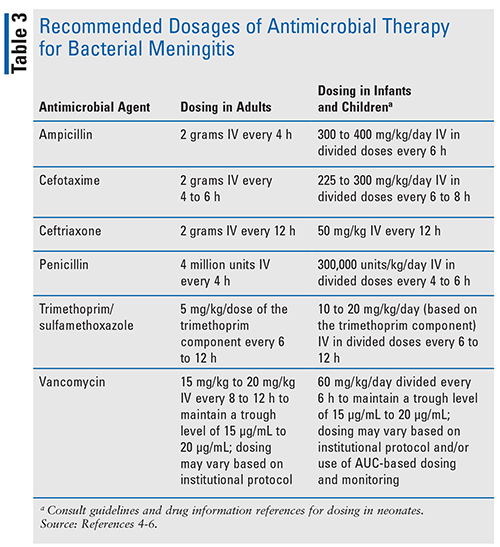
Once the causative microorganism has been identified and the results of susceptibility tests are known, antimicrobial therapy should be modified as indicated in Table 2.4-6,11 Recommended dosing of commonly used agents is included in Table 3.4-6
Adjunctive Therapy: Role of Dexamethasone and Rifampin
The rationale for use of adjunctive dexamethasone is to reduce the inflammatory response in the subarachnoid space (caused by the release of bacterial components from bactericidal therapy) that leads to inflammation-related neurological complications.2,11 Adjunctive dexamethasone has been shown to decrease mortality in adults with pneumococcal meningitis and to decrease hearing loss in children with meningitis caused by Haemophilus influenzae type b.6,11 As such, the Infectious Diseases Society of America guidelines recommend the use of adjunctive dexamethasone in adults with suspected or proven meningitis caused by Streptococcus pneumoniae and in infants (not neonates) and children with meningitis caused by H influenzae type b.6,11 The recommended dosing regimen of dexamethasone is 0.15 mg/kg every 6 hours for 2 to 4 days (typically a 4-day duration), with the first dose given 10 to 20 minutes before or at the same time as the first dose of antimicrobial therapy.5,6 If testing reveals another pathogen or if bacterial meningitis is ruled out, dexamethasone should be discontinued, because there has been no proven, consistent benefit from its use for the treatment of other pathogens.6 Additionally, dexamethasone should not be given to those who have already received antimicrobial therapy, as this is unlikely to improve outcomes.6
If dexamethasone is given to a patient with suspected pneumococcal meningitis, some experts add rifampin to the empiric antimicrobial regimen of vancomycin in combination with a third-generation cephalosporin, either as part of the initial regimen or pending the results of susceptibility testing. For isolates of S pneumoniae with resistance to a cephalosporin (defined as an MIC >2 mcg/mL), rifampin may be continued or added because it has been shown to be synergistic with ceftriaxone against cephalosporin-resistant S pneumoniae.6,12
PREVENTION
Chemoprophylaxis
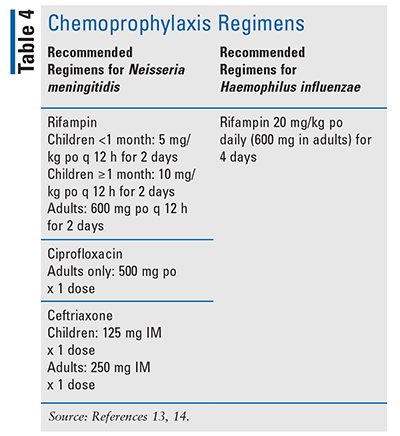
Antimicrobial chemoprophylaxis (Table 4) is indicated in certain situations to prevent the spread of infection from those with meningitis caused by N meningitidis or H influenzae.13,14
Chemoprophylaxis against meningococcal infection is indicated for a close contact, defined as prolonged contact ( ≥8 hours) in close proximity ( ≥3 feet), or anyone who has been directly exposed to the patient’s oral secretions in the 7 days before the onset of the infected patient’s symptoms. Thus, chemoprophylaxis against N meningitidis may be used for household members, child-care center contacts, or anyone with direct exposure to an infected patient’s oral secretions from activities such as kissing, mouth-to-mouth resuscitation, endotracheal intubation, or endotracheal tube management. Antimicrobial chemoprophylaxis for N meningitidis should be administered as early as possible and ideally within 24 hours after identification of the index patient. It is not recommended to administer prophylaxis if it has been more than 14 days after exposure, since it is likely of limited or no value after this time frame.13
Chemoprophylaxis against H influenzae type b is recommended for all household contacts in households with individuals younger than age 4 years who are not fully vaccinated; for any immunocompromised household contacts who are younger than age 18 years (regardless of their vaccination status); and for all attendees and providers in child-care settings when two or more cases of invasive infection have occurred within 60 days and there are children who attend the facility who have not received the complete vaccine series.14
Vaccines
There are vaccines that target selected bacterial etiologies of bacterial meningitis: H influenzae, S pneumoniae, and N meningitidis. The CDC Advisory Committee on Immunization Practices (ACIP) recommends H influenzae type b (Hib) vaccination as part of routine childhood immunizations and in those with anatomical or functional asplenia (including sickle cell disease) who have not previously received the vaccine, those undergoing elective splenectomy (some experts recommend giving it only to those who are unimmunized, while others recommend its use regardless of vaccine history), and recipients of hematopoietic stem-cell transplant.14-16 In the U.S., there are three monovalent conjugate Hib vaccines (ActHIB, Hiberix, and PedvaxHIB) that may be used in pediatric and adult patients and one pediatric combination vaccine (Pentacel) which provides protection against Hib, diphtheria, tetanus, pertussis, and polio.17
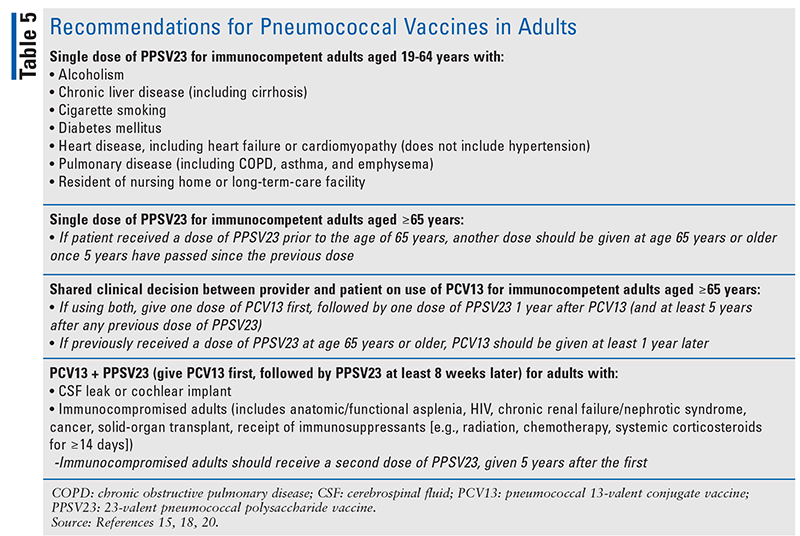
Two vaccines are currently marketed for the prevention of pneumococcal disease: the pneumococcal 13-valent conjugate vaccine (PCV13 or Prevnar 13) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23 or Pneumovax 23).18 PCV13 protects against 13 serotypes of S pneumoniae while PPSV23 protects against 23 serotypes.19 PCV13 is recommended as a four-dose series as part of routine childhood immunizations for all children younger than age 5 years and as a single dose for those aged 6 years and older with risk factors for pneumococcal disease. These risk factors in children are chronic heart disease; chronic lung disease; diabetes mellitus; CSF leaks; cochlear implant; anatomic or functional asplenia (including sickle cell disease); or immunocompromising conditions (including HIV, chronic renal failure/nephrotic syndrome; diseases being treated with immunosuppressive drugs or radiation; solid organ transplant; and congenital or acquired immunodeficiency).16,18
Table 5 includes recommendations on the use of pneumococcal vaccines in adults.15,18,20 Although the ACIP previously recommended routine administration of both PCV13 and PPSV23 to all individuals aged 65 years and older, the ACIP modified its recommendations in June 2019. The updated recommendations state that PCV13 should not automatically be given to all immunocompetent individuals aged 65 years and older but should be a part of “shared clinical decision- making” between the patient and provider.15,20
There are two types of meningococcal vaccines available in the U.S.: quadrivalent conjugate meningococcal vaccines that protect against serogroups A, C, W, and Y of N meningitidis and vaccines that protect against serogroup B (Table 6).21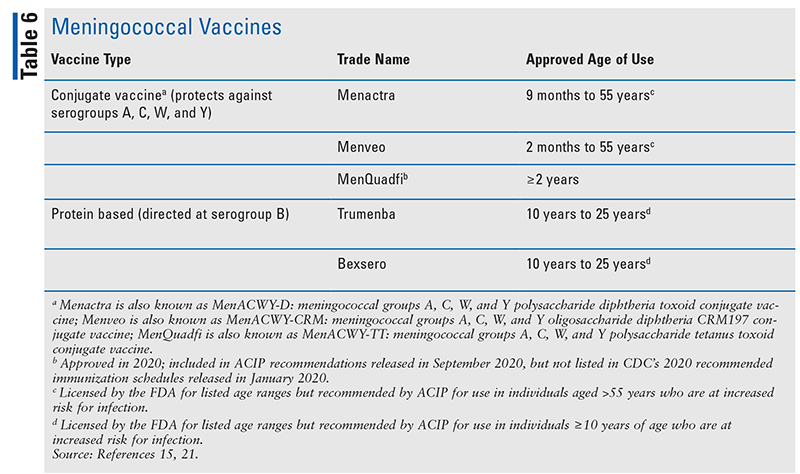
A quadrivalent conjugate (MenACWY) vaccine is recommended by the ACIP as routine vaccination for adolescents aged 11 or 12 years and older (with a booster dose at age 16 years) and for those aged 2 months and older who are at increased risk for meningococcal disease. These risk factors include anatomic or functional asplenia, complement component deficiency, use of a complement inhibitor (e.g., eculizumab or ravulizumab), and HIV infection. Also at increased risk are microbiologists with routine exposure to isolates, persons at increased risk during an outbreak, those who travel to or live in countries in which meningococcal disease is hyperendemic or epidemic, military recruits, and first-year college students living in residence halls (unless they received a dose on or after the age of 16 years and it has not been more than 5 years, or as required by institutional policy). Booster doses are recommended for those who remain at increased risk for infection. The dosing schedule and timing of booster dose for MenACWY vaccines varies according to age, indication for use, and age at receipt of previous vaccination. According to the ACIP, the same product is recommended, but not required, for all doses.21
In addition, ACIP recommends routine use of a serogroup B meningococcal (MenB) vaccine series among persons aged 10 years and older who are at increased risk for serogroup B meningococcal disease. These risks include those with persistent complement component deficiencies or who are receiving a complement inhibitor; anatomic or functional asplenia; microbiologists with routine exposure; and those considered to be at increased risk during an outbreak caused by serogroup B. Booster doses are recommended for those who remain at increased risk for infection. In addition, a MenB series may be used on the basis of “shared clinical decision-making” to provide short-term protection for adolescents and young adults aged 16 to 23 years who are not at increased risk for infection. Serogroup B vaccines are not interchangeable, and the same product should be used for all doses in the series.16,21
Role of Immunizations During the COVID-19 Pandemic
According to the CDC, routine vaccination is an essential preventive service that protects individuals and communities from vaccine-preventable diseases and outbreaks and may help them avoid unnecessary medical visits and hospitalizations. As such, routine vaccines should not be delayed just because of the COVID-19 pandemic, and providers should follow the recommended CDC immunization schedules. However, the CDC recommends that routine vaccination be deferred in certain situations in order to avoid exposing healthcare workers and/or other patients to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Routine vaccinations should be deferred for 10 days after a positive test result for asymptomatic and presymptomatic persons who have tested positive for SARS-CoV-2. Vaccination should also be deferred for persons with suspected or confirmed COVID-19 who are symptomatic until all four criteria to discontinue isolation have been met (at least 10 days after onset of symptoms, absence of fever for 24 hours without the use of fever-reducing medications, improving symptoms of COVID-19, and the person is no longer considered moderately to severely ill). Additionally, persons who have had a known exposure to COVID-19 should be advised not to seek outpatient care solely for the purpose of receiving vaccines until after the end of their quarantine period.22
Pharmacist’s Role
Pharmacists play an integral role in the management of bacterial meningitis by working collaboratively with clinicians to select the most appropriate empiric and directed antimicrobial therapy regimen based on patient-specific factors. Pharmacists can recommend chemoprophylaxis when warranted and advocate for routine use of vaccinations for prevention of this serious infection.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. CDC. Bacterial meningitis. www.cdc.gov/meningitis/bacterial.html. Accessed October 30, 2020.
2. van de Beek D, Brouwer M, Hasbun R, et al. Community-acquired bacterial meningitis. Nat Rev Dis Primers. 2016;2:16074.
3. Wright WF, Pinto CN, Palisoc K, Baghli S. Viral (aseptic) meningitis: a review. J Neurol Sci. 2019;398:176-183.
4. Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 Infectious Diseases Society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017;64(6):e34-e65.
5. van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22(Suppl 3):S37-S62.
6. Tunkel A, Hartman B, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.
7. Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47(3):303-327.
8. van de Beek D, Brouwer MC, Thwaites GE, Tunkel AR. Advances in treatment of bacterial meningitis. Lancet. 2012;380(9854):1693-1702.
9. CDC. Haemophilus influenzae disease (including Hib). www.cdc.gov/hi-disease/clinicians.html. Accessed November 2, 2020.
10. van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351(18):1849-1859.
11. Hasbun R. Update and advances in community acquired bacterial meningitis. Curr Opin Infect Dis. 2019;32(3):233-238.
12. Costerus JM, Brouwer MC, Bijlsma MW, van de Beek D. Community-acquired bacterial meningitis. Curr Opin Infect Dis. 2017;30(1):135-141.
13. Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Com-mittee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-2):1-28.
14. Briere EC, Rubin L, Moro PL, et al. Prevention and control of Haemophilus influenzae type b disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-01):1-14.
15. CDC. Recommended adult immunization schedule for ages 19 years or older, United States 2020. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed September 18, 2020.
16. CDC. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2020. www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf.
17. CDC. About Hib vaccines. www.cdc.gov/vaccines/vpd/hib/hcp/about-vaccine.html. Accessed September 18, 2020.
18. CDC. Pneumococcal vaccination: summary of who and when to vaccinate. www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Accessed September 18, 2020.
19. CDC. About pneumococcal vaccines. www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed November 2, 2020.
20. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ³65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
21. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69(No. RR-9):1-41.
22. CDC. Interim guidance for routine and influenza immunization services during the COVID-19 pandemic. www.cdc.gov/vaccines/pandemic-guidance/index.html. Accessed November 2, 2020.
To comment on this article, contact rdavidson@uspharmacist.com.