US Pharm. 2007;32(5):HS-32-HS-43.
Infectious endocarditis (IE)
is a relatively uncommon infection that was first described in 1885 by William
Osler.1 Although IE is somewhat rare, the condition continues to
have a mortality rate of approximately 40%.2,3 Depending on the
severity and rapidity of onset of symptoms, IE can be classified as acute
or subacute.4 The typical presentation of IE comprises
fever, weight loss, and fatigue; almost all patients present with a heart
murmur.
According to the Duke
criteria, a diagnostic tool for IE, certain symptoms and laboratory and
echocardiographic findings can be categorized as major or minor criteria.
5-7 Major criteria include a positive result from two separate blood
cultures as well as the presence of a vegetation, visualized via
transesophageal echocardiogram or transthoracic echocardiogram. Minor criteria
include a predisposing heart condition, fever, vascular and immunologic
findings (e.g., Janeway's lesions or Osler's nodes and Roth spots,
respectively), and a positive blood culture that does not meet the major
criteria. Janeway's lesions are reddened lesions on the palms and soles of the
feet, whereas Osler's nodes are nodules on the surface of the fingertips and
toes.4 Roth spots are an ophthalmologic phenomenon involving
retinal hemorrhages.8
The diagnosis of IE can be
categorized as definite, possible, or rejected, depending
on the presence of major or minor criteria. A diagnosis of definite IE
requires either pathologic evidence of a vegetation or a combination of two
major criteria, one major and three minor criteria, or five minor criteria.
Possible IE requires either two criteria (one major and one minor) or three
minor criteria. IE can be rejected for any of the following reasons: an
alternative diagnosis is determined; there is no pathologic support for the
findings of IE; the patient defervesces with antibiotic treatment in less than
four days with resolution of the manifestations of IE; or the patient does not
meet any Duke criteria.5-7,9
The pathogenesis of IE
involves clot formation, as a result of blood and "subendothelial" particles,
on one of the heart valves followed by binding of pathogens from bacteremia to
the clot. An immunologic response is then initiated involving cytokines and
white blood cells that results in the formation of a vegetation.10,11
Usually, patients who develop IE have underlying risk factors, such as the
presence of a prosthetic heart valve, rheumatic heart disease, mitral valve
prolapse, hemodialysis, diabetes, HIV, inadequate dental care, and intravenous
drug abuse.4,12-21
The most common bacterial
pathogens implicated in IE are Streptococcus and Staphylococcus
species, accounting for up to 80% to 90% of cases, while
Enterococcus species are the third most common bacterial etiology.
22-26 Other causative organisms are the HACEK organisms (Haemophilus
influenzae, Haemophilus parainfluenzae, Haemophilus aphrophilus, Haemophilus
paraphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis,
Eikenella corrodens, Kingella kingae, and Kingella denitrificans),
Enterobacteriaceae, Pseudomonas species, Neisseria gonorrhoeae,
and fungi.7 Although blood cultures are usually positive,
culture-negative IE can occur in up to 20% of patients.27
Pharmacologic Treatment of
Common Organisms
Initiating the
appropriate treatment in patients is critical because of the high mortality
rate associated with IE. This article focuses on the treatment of IE according
to the American Heart Association (AHA) guidelines for the most common
organisms: viridans group streptococci, the Staphylococcus
species, and the Enterococcus species.7 In addition,
daptomycin, a new treatment option for staphylococcal IE, is reviewed.
The treatment of IE is highly
dependent on whether the patient has a native or prosthetic valve. Not only is
the treatment slightly different, but the duration of treatment is often
longer for patients with a prosthetic valve.7
Viridans Group
Streptococci: The
viridans group streptococci are also known as alpha-hemolytic streptococci
and are common causes of IE in patients with native valves.7 Common
inhabitants of the oral cavity, these gram-positive organisms can
become pathogenic causes of IE in patients who require dental and/or gingival
treatment and have a history of IE or in those who have had cardiac valve
surgery.2,28 In the viridans group streptococci, bacteria
frequently associated with IE include Streptococcus anguis, Streptococcus
oralis (mitis), Streptococcus salivarius, Streptococcus mutans, and
Gemella morbillorum (formerly Streptococcus morbillorum). In
addition, Streptococcus bovis, a group D streptococcus, is another
common pathogen of IE.
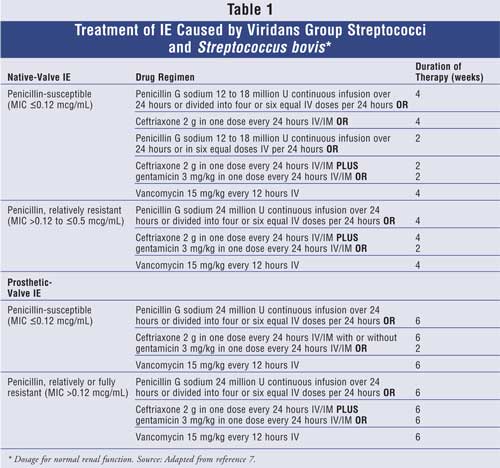
The treatment of IE caused by these
organisms involves classifying the bacteria as highly penicillin-susceptible
(minimum inhibitory concentration [MIC] £0.12 mcg/mL) or relatively/fully
penicillin-resistant (MIC >0.12 mcg/mL). In both native- and prosthetic-valve
IE, penicillin and ceftriaxone are considered the drugs of choice, and
gentamicin may be added for synergy. The duration of treatment depends on
whether the valve is native or prosthetic. If gentamicin is used, it is
important to achieve peak and trough serum concentrations of 3 to 4 mcg/mL and
less than 1 mcg/mL, respectively.7 Vancomycin can be considered in
patients unable to take a beta-lactam antibiotic. It should be noted that
target peak and trough concentrations for vancomycin are 30 to 45 mcg/mL and
10 to 15 mcg/mL, respectively.7 For IE patients with a native
valve, the duration of treatment is two to four weeks depending on the drug
regimen and MIC of the organism. However, six weeks of treatment is required
for prosthetic valve disease. Table 1 describes the appropriate dosages
and duration of therapy according to the type of valve involved.
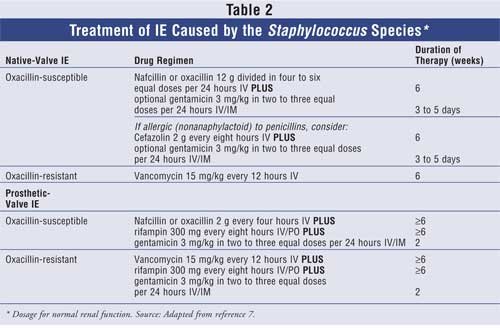
Staphylococcus Species:
Recently, staphylococcal infections in general have increased significantly in
both the inpatient and the outpatient settings.29-31 Common
causative staphylococcal organisms of IE include Staphylococcus aureus
(coagulase-positive) and Staphylococcus epidermidis
(coagulase-negative), correlating most commonly to native-valve and
prosthetic-valve IE, respectively.7 The treatment of staphylococcal
IE involves determining whether the pathogen is oxacillin-susceptible or
oxacillin-resistant. If susceptible, nafcillin or oxacillin are the preferred
agents, with the addition of gentamicin or rifampin depending on the presence
of a prosthetic valve. For native-valve patients who have a "nonanaphylactoid"
penicillin allergy, cefazolin with or without gentamicin may be considered.
For native-valve patients with oxacillin-resistant strains, vancomycin is
considered first-line therapy; however, the addition of rifampin and
gentamicin to the vancomycin regimen is recommended for patients with a
prosthetic valve. A minimum of six weeks of therapy is required, although as
with viridans streptococci, the exact duration of treatment is determined
based on the type of valve involved. In addition, target vancomycin and
gentamicin peak and trough concentrations are identical to viridans
streptococci treatment.7 Further details of treatment are described
in Table 2.
Daptomycin:
Daptomycin (Cubicin), a cyclic lipopeptide with concentration-dependent
bactericidal activity against S aureusand other gram-positive
organisms, including vancomycin-resistant Enterococcus (VRE) and
methicillin-resistant S aureus (MRSA), was recently approved by the FDA
for the treatment of bacteremia and right-sided endocarditis caused by S
aureus (both methicillin-susceptible S aureus [MSSA] and MRSA).
32-35 Based on a randomized, controlled, multinational, open-label
study, daptomycin was considered noninferior to standard therapy with
comparable success rates. In this clinical study, daptomycin (6 mg/kg every 24
hours) was compared to standard treatment, which consisted of either an
antistaphylococcal penicillin (nafcillin, oxacillin, or flucloxacillin 2 g
every four hours) or vancomycin (1 g every 12 hours) (based on susceptibility
testing) plus gentamicin (1 mg/kg every eight hours) in the treatment of S
aureus bacteremia and endocarditis.34 Of the 236 patients
randomized, 181 were diagnosed as having definite or possible IE at baseline.
Of these patients, only 53 were determined to have staphylococcal IE; 35
patients had right-sided IE, and 18 patients had left-sided IE. The overall
incidence of MRSA was 38%. The primary outcome of the study was the clinical
success rate six weeks after the last treatment dose. For patients with
uncomplicated right-sided IE (n = 10), success occurred in 50% (three of six
patients) versus 25% (one of four patients) of patients receiving daptomycin
and standard therapy, respectively (absolute difference, 25%; 95% confidence
interval [CI], –33.3 to 83.3). Patients with complicated right-sided IE (n =
25) had a 38.5% response in the daptomycin arm versus a 50% response in the
standard therapy arm (absolute difference, –11.5%; 95% CI, –50.3 to 27.2).
Success rates for patients with left-sided IE were 11.1% (one of nine
patients) for daptomycin versus 22.2% (two of nine patients) in patients
receiving standard therapy (absolute difference, –11.1%; 95% CI, –45.2 to
22.9) and was lower in comparison to right-sided IE patients. Treatment
failure (combined data of patients with IE or bacteremia) due to relapsing or
persisting S aureus infections was 15.8% and 9.6%, respectively for
daptomycin and the standard therapy arm (P = .17). Six out of the 19
patients in the daptomycin failure group (32%) had isolates that developed
resistance to the drug while on therapy (MIC ?2 mcg/mL). Overall
survival rates were similar between the groups (10.8% mortality rate with
daptomycin compared with 11.3% mortality rate for those receiving standard
therapy). The incidence of adverse effects was also comparable between the
groups, 35% (daptomycin) versus 42.2% (standard therapy) (P = .29),
with the most common events in both groups being gastrointestinal complaints
and anemia. Creatine kinase (CK) elevations were more common in the daptomycin
group (6.7% vs. 0.9%; P = .04); however, only three patients had to
discontinue therapy as a result. Therefore, based on the results of this
study, daptomycin may be considered an option--although probably not
first-line--for patients with right-sided IE caused by S aureus
(MRSA or MSSA).34
Dosing recommendations for
daptomycin for the treatment of IE/bacteremia is 6 mg/kg every 24 hours, with
dosing adjustments necessary in patients with a creatinine clearance of less
than 30 mL/minute (estimated by the Cockcroft-Gault equation) and those who
are dependent on dialysis (6 mg/kg every 48 hours).34,35 Clinicians
should be aware of the need to monitor patients for the development of muscle
pain or weakness, especially in patients who have renal insufficiency or who
are taking concomitant statin therapy. Temporarily discontinuing statin
therapy should be considered if deemed necessary in some patients.
Additionally, creatine phosphokinase should be monitored weekly while on
therapy and possibly more often in patient populations at an increased risk
for rhabdomyolysis.32,35 Furthermore, patients who do not seem to
respond to daptomycin should have their treatment evaluated due to the
potential emergence of resistance to the antimicrobial. At this time, there
are limited data for the use of daptomycin in patients with prosthetic-valve
endocarditis (not included in the study) and left-sided IE (small number of
patients in the study). Further investigation is needed in those patient
populations before routine use of daptomycin can be recommended.34,35
Enterococcus Species
: Enterococcus, a
gram-positive organism, is considered to be part of the normal flora of the
gastrointestinal tract and sometimes the anterior urethra.24 IE
associated with the Enterococcus species often occurs in patients with
a history of genitourinary or obstetrical procedures.26 There are
over 15 different species of Enterococcus, of which Enterococcus
faeciumand Enterococcus faecalis are most commonly associated with
IE.2,7,24,26 Of the two species, E faecium is often more
resistant in comparison to E faecalis, but it causes IE less frequently.
24 Enterococcal IE is associated with high relapse rates, and,
therefore, susceptibility testing (MIC determination) to
ampicillin/penicillin, vancomycin, and aminoglycosides (specifically,
gentamicin or streptomycin) should be performed.7,26,36
In contrast to the
Staphylococcus and Streptococcus species, the drug therapy
for enterococcal IE is the same regardless of the presence or absence of a
prosthetic valve. Treatment often involves the use of a cell-wall active drug
such as a beta-lactam (ampicillin or penicillin) or vancomycin in combination
with an aminoglycoside (gentamicin or streptomycin) for synergy. Synergy is
needed with an aminoglycoside in order to achieve a bactericidal effect
because most cell-wall active drugs are only considered bacteriostatic against
enterococci.2,7,26,37 Of the two aminoglycosides used to
treat enterococcal endocarditis, gentamicin is primarily used unless
resistance is present.7 Other aminoglycosides such as tobramycin
and amikacin are not used in enterococcal infections due to the presence of
intrinsic resistance.7 Aminoglycoside dosing should be based on the
patient's renal function. Gentamicin levels should target peaks of 3 to 4
mcg/mL and troughs of less than 1 mcg/mL. Streptomycin dosing should target
peaks of 20 to 35 mcg/mL and trough concentrations of 10 mcg/mL.7
Currently, once-daily or consolidated aminoglycoside dosing is not recommended
in the treatment of enterococcal IE due to the lack of clinical evidence.
7,26 In terms of duration of treatment, patients with a prosthetic valve
who are receiving vancomycin-aminoglycoside combination therapy (less active
in comparison to ampicillin/penicillin combinations with aminoglycoside) and
those who have had symptoms for longer than three months should be treated for
a minimum of six weeks. Similarly, patients with VRE not susceptible to
ampicillin should be treated for at least eight weeks.7
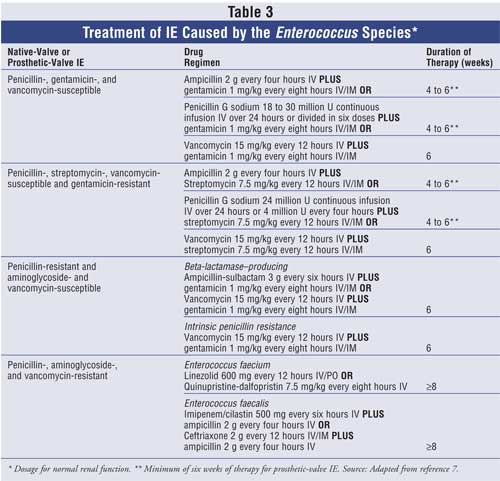
The treatment of enterococcal
IE depends on susceptibilities to ampicillin (or penicillin), gentamicin (or
streptomycin), and vancomycin (see Table 3). For patients with
enterococcal IE that is susceptible to ampicillin/penicillin, gentamicin, and
vancomycin, recommended treatment is with ampicillin or penicillin G plus
gentamicin for four to six weeks. Vancomycin (goal peak 30 to 45 mcg/mL and
trough 10 to 15 mcg/mL) should be used only in patients with allergies to
beta-lactam therapy or in those patients with enterococcal infection that is
penicillin-resistant (MIC >16 mcg/mL); however, treatment must be for six
weeks in these cases. If the Enterococcus species is resistant to
gentamicin but susceptible to streptomycin, then streptomycin can be used in
place of gentamicin. Beta-lactamase–producing strains of the
Enterococcus species can be treated with ampicillin-sulbactam (or
vancomycin) plus gentamicin for six weeks. Conversely, strains resistant to
gentamicin will require more than six weeks of therapy.7
For patients with enterococcal
strains that are multidrug resistant, including reduced susceptibility to
penicillins, aminoglycosides, and vancomycin therapy, drug options should be
guided based on the specific species of Enterococcus; in addition,
the treatment duration is usually eight to 12 weeks.7 These types
of organisms are often challenging to treat due to their resistance against
multiple antibiotics. VRE can be occasionally sensitive to ampicillin, and in
these cases it is recommended to use ampicillin/penicillin (in combination
with an aminoglycoside if sensitive). Newer antienterococcal agents such as
quinupristin-dalfopristin, which is effective only against E faecium,
and linezolid, which is effective against both E faecium and E
faecalis, can be used to treat VRE that is not susceptible to
ampicillin/penicillin. However, neither agent is likely to be curative in
nature due to bacteriostatic effects.7 Although daptomycin may have
a role in VRE endocarditis, further investigation for this use is needed.
32,38 For VRE strains that are E faecium and not susceptible to
ampicillin, linezolid or quinupristin-dalfopristin may be considered for
treatment. VRE strains that are E faecalis and not susceptible to
ampicillin can be treated with double-beta lactam combinations of
imipenem-cilastin plus ampicillin or ceftriaxone plus ampicillin due to the
presence of synergistic bactericidal activity between these agents.
7,26,39,40
Role of the Pharmacist
The treatment of IE involves prompt
and appropriate pharmacologic therapy, although surgery may be required.
Pharmacists have a vital role in preventing certain adverse effects of
medications, as well as in ensuring optimal therapeutic efficacy. When using
penicillin, the time-dependent killing nature of the antibiotic can be
maximized by continuous infusions or by ensuring that the doses are given
every four to six hours. In addition, monitoring of vancomycin and
aminoglycoside concentrations can help avoid the side effects of
nephrotoxicity and ototoxicity and ensure the efficacy of these drugs. With
daptomycin, careful monitoring of CK serum concentrations and screening for
concurrent statin use are also required to prevent cases of rhabdomyolysis.
Regarding the use of rifampin, pharmacists should counsel patients on
potential drug interactions.
Summary
IE continues to be a
life-threatening infection that often requires a prolonged duration of
antibiotic therapy and sometimes surgery in order to be treated appropriately.
The most common organisms causing IE are Streptococcus,
Staphylococcus, and Enterococcus species. The infecting organism,
susceptibility patterns, and AHA guideline recommendations should be
considered to guide antibiotic therapy as well as the duration of treatment.
Newer treatment options for drug-resistant organisms such as MRSA and VRE need
to be added to the repertoire of drugs that are currently available for the
treatment of IE. However, further research on these agents is needed to
establish their safety and efficacy for use in this setting.
References
1. Osler W. Gulstonian lectures on malignant endocarditis. Lecture I. 1,
415-418. 1885.
2. Hoen B. Epidemiology and antibiotic treatment of infective endocarditis: an
update. Heart. 2006;92:1694-1700.
3. Cabell CH, Jollis JG, et al. Changing patient characteristics and the
effect on mortality in endocarditis. Arch Intern Med. 2002;162:90-94.
4. Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J
Med. 2001;345:1318-1330.
5. Durack DT, Lukes AS, et al. New criteria for diagnosis of infective
endocarditis: utilization of specific echocardiographic findings. Duke
Endocarditis Service. Am J Med. 1994;96:
200-209.
6. Li JS, Sexton DJ, et al. Proposed modifications to the Duke criteria for
the diagnosis of infective endocarditis. Clin Infect Dis.
2000;30:633-638.
7. Baddour LM, Wilson WR, et al. Infective endocarditis: diagnosis,
antimicrobial therapy, and management of complications: a statement for
healthcare professionals from the Committee on Rheumatic Fever, Endocarditis,
and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the
Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and
Anesthesia, American Heart Association: endorsed by the Infectious Diseases
Society of America. Circulation. 2005;111:e394-e434.
8. Ling R, James B. White-centred retinal haemorrhages (Roth spots).
Postgrad Med J. 1998;74:581-582.
9. Kanafani ZA, Fowler VG, Jr. [Staphylococcus aureus infections: new
challenges from an old pathogen]. Enferm Infec Microbiol Clin.
2006;24:182-193.
10. Beynon RP, Bahl VK, et al. Infective endocarditis. BMJ.
2006;333:334-339.
11. Moreillon P, Que YA. Infective endocarditis. Lancet.
2004;363:139-149.
12. Hogevik H, Olaison L, et al. Epidemiologic aspects of infective
endocarditis in an urban population. A 5-year prospective study. Medicine.
1995;74:324-339.
13. Watanakunakorn C, Burkert T. Infective endocarditis at a large community
teaching hospital, 1980-1990. A review of 210 episodes. Medicine.
1993;72:90-102.
14. Frontera JA, Gradon JD. Right-side endocarditis in injection drug users:
review of proposed mechanisms of pathogenesis. Clin Infect Dis.
2000;30:374-379.
15. Strom BL, Abrutyn E, et al. Risk factors for infective endocarditis: oral
hygiene and nondental exposures. Circulation. 2000;102:2842-2848.
16. Manoff SB, Vlahov D, et al. Human immunodeficiency virus infection and
infective endocarditis among injecting drug users. Epidemiology.
1996;7:566-570.
17. Ribera E, Miro JM, et al. Influence of human immunodeficiency virus 1
infection and degree of immunosuppression in the clinical characteristics and
outcome of infective endocarditis in intravenous drug users. Arch Intern Med
. 1998;158:2043-2050.
18. Zuppiroli A, Rinaldi M, et al. Natural history of mitral valve prolapse.
Am J Cardiol. 1995;75:1028-1032.
19. Jalal S, Khan KA, et al. Clinical spectrum of infective endocarditis: 15
years experience. Indian Heart J. 1998;50:516-519.
20. Choudhury R, Grover A, et al. Active infective endocarditis observed in an
Indian hospital 1981-1991. Am J Cardiol. 1992;70:1453-1458.
21. Bonow RO, Carabello BA, et al. ACC/AHA 2006 guidelines for the management
of patients with valvular heart disease: a report of the American College of
Cardiology/American Heart Association Task Force on Practice Guidelines
(writing committee to revise the 1998 Guidelines for the Management of
Patients With Valvular Heart Disease): developed in collaboration with the
Society of Cardiovascular Anesthesiologists: endorsed by the Society for
Cardiovascular Angiography and Interventions and the Society of Thoracic
Surgeons. Circulation. 2006;114:ee84-231.
22. Venezio FR, Westenfelder GO, et al. Infective endocarditis in a community
hospital. Arch Intern Med. 1982;142:789-792.
23. Kim EL, Ching DL, et al. Bacterial endocarditis at a small community
hospital. Am J Med Sci. 1990;299:87-93.
24. Megran DW. Enterococcal endocarditis. Clin Infect Dis.
1992;15:63-71.
25. Fowler VG Jr, Olsen MK, et al. Clinical identifiers of complicated
Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163:2066-2072.
26. Bashore TM, Cabell C, et al. Update on infective endocarditis. Curr
Probl Cardiol. 2006;31:274-352.
27. Werner M, Andersson R, et al. A clinical study of culture-negative
endocarditis. Medicine. (Baltimore) 2003;82:263-273.
28. Gould FK, Elliott TS, et al. Guidelines for the prevention of
endocarditis: report of the Working Party of the British Society for
Antimicrobial Chemotherapy. J Antimicrob Chemother. 2006;57:1035-1042.
29. Wisplinghoff H, Bischoff T, et al. Nosocomial bloodstream infections in US
hospitals: analysis of 24,179 cases from a prospective nationwide surveillance
study. Clin Infect Dis. 2004;39:309-317.
30. Friedman ND, Kaye KS, et al. Health care–associated bloodstream infections
in adults: a reason to change the accepted definition of community-acquired
infections. Ann Intern Med. 2002;137:791-797.
31. Naimi TS, LeDell KH, et al. Comparison of community- and health
care-associated methicillin-resistant Staphylococcus aureus infection. JAMA
. 2003;290:2976-2984.
32. Schriever CA, Fernandez C, et al. Daptomycin: a novel cyclic lipopeptide
antimicrobial. Am J Health Syst Pharm. 2005;62:1145-1158.
33. Steenbergen JN, Alder J, et al. Daptomycin: a lipopeptide antibiotic for
the treatment of serious Gram-positive infections. J Antimicrob Chemother
. 2005;55:283-288.
34. Fowler VG, Jr., Boucher HW, et al. Daptomycin versus standard therapy for
bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med
. 2006;355:653-665.
35. Cubicin (daptomycin) [package insert]. Lexington MCP. 2006.
36. Hricak V Jr, Kovacik J, et al. Endocarditis due to enterococcus faecalis:
risk factors and outcome in twenty-one cases from a five year national survey.
Scand J Infect Dis. 1998;30:540-541.
37. Krogstad DJ, Pargwette AR. Defective killing of enterococci: a common
property of antimicrobial agents acting on the cell wall. Antimicrob Agents
Chemother. 1980;17:965-968.
38. Segreti JA, Crank CW, et al. Daptomycin for the treatment of gram-positive
bacteremia and infective endocarditis: a retrospective case series of 31
patients. Pharmacotherapy. 2006;26:347-352.
39. Brandt CM, Rouse MS, et al. Effective treatment of multidrug-resistant
enterococcal experimental endocarditis with combinations of cell wall-active
agents. J Infect Dis. 1996;173:909-913.
40. Gavalda J, Torres C, et al. Efficacy of ampicillin plus ceftriaxone in
treatment of experimental endocarditis due to Enterococcus faecalis strains
highly resistant to aminoglycosides. Antimicrob Agents Chemother.
1999;43:639-646.
To comment on this article, contact
editor@uspharmacist.com.





