US Pharm. 2017;42(7)(Specialty&Oncology suppl):16-19.
ABSTRACT: Asthma is a common, chronic respiratory disease in which the lung’s airways become inflamed and narrowed. While the majority of patients with asthma can be treated effectively with the proper use of maintenance medications, patients with severe asthma are refractory to current standards of treatment, including oral corticosteroids. Eosinophilic asthma is a subset of severe asthma that is characterized by increased eosinophil counts. Because increased eosinophilia correlates with worse disease, mediators of the eosinophil pathway, such as interleukin-5 (IL-5), are targets for preventing eosinophil-mediated inflammation. Recently, the new IL-5 receptor antagonists reslizumab and mepolizumab were approved by the FDA for use in severe eosinophilic asthma. Used as maintenance therapy, these agents may benefit patients with eosinophilic asthma.
Asthma is a common, chronic respiratory disease in which the lung’s airways become inflamed and narrowed. This reversible disease of chronic airway inflammation, which affects 5% to 10% of the population in developed countries, is associated with airway hyperresponsiveness.1 While the majority of patients with asthma can be treated effectively with the proper use of maintenance medications, approximately 10% to 20% of patients are refractory to current standards of treatment—including oral corticosteroids—and are considered to have severe asthma.
The U.S. Department of Health and Human Services’ Asthma Care: Quick Reference summarizes the Global Strategy for Asthma Management and Prevention guidelines, which includes easy-to-use tables for classifying asthma severity and control, as well as a stepwise approach to managing asthma.2 In 2014, the European Respiratory Society/American Thoracic Society Task Force on severe asthma updated the definition of severe asthma and discussed its phenotypes.3 By definition, severe asthma requires treatment with guideline-based medications—such as high-dose inhaled corticosteroids (ICS) in combination with a long-acting beta-agonist (LABA) or leukotriene modifier/theophylline in the previous year or systemic corticosteroids for >50% of the previous year—to prevent the patient’s symptoms from becoming or remaining uncontrolled.3
Eosinophils, the major inflammatory cells involved in asthma pathophysiology, are considered a biomarker for asthma.4 Eosinophils release inflammatory mediators, leading to inflamed and narrowed airways in patients with asthma. Eosinophilic inflammation correlates with asthma severity.5 In eosinophilic asthma, a subphenotype of severe asthma, a patient’s eosinophil levels are elevated despite compliance with adequate dosing of ICS. Because interleukin (IL)-5 is involved in eosinophil differentiation, maturation, recruitment, and activation, it has been identified as an important target for new biologics.6 Recently, two IL-5 receptor antagonists—reslizumab and mepolizumab—have been approved for severe eosinophilic asthma.7,8 Effective IL-5 antagonism has been demonstrated to provide control in eosinophilic asthma, thereby acting as a substitute for oral corticosteroids.6
Traditional Therapy
The goals of asthma therapy include long-term symptom control, maintenance of normal activity levels, minimization of the risk of exacerbations, and reduction of the risk of adverse events. Treatment is individualized to fit the patient’s specific goals and asthma severity. Pharmacologic options include rescue medications and maintenance medications.
Rescue Medications: Rescue medications are employed infrequently for quick relief of asthma symptoms. The most effective and widely used rescue medication is a short-acting beta-agonist, such as albuterol.1,9 Oral and injectable corticosteroids may be taken short-term, typically for 3 to 10 days, for asthma exacerbations. Frequent albuterol use or exacerbations requiring frequent corticosteroid use necessitate the use of maintenance medications.1
Maintenance Medications: Maintenance medications are used regularly and continuously for effective long-term treatment of asthma. They are used to control symptoms and reduce the risk of future exacerbations, but they do not typically provide immediate symptom relief. Early initiation of daily maintenance medication has been associated with improved clinical outcomes. Patient-specific factors determine which maintenance medication(s) should be prescribed. A tiered stepwise approach to therapy is recommended based on asthma severity and control. The step at which therapy is initiated corresponds to asthma severity at the time of diagnosis; therapy is subsequently stepped up if the disease is uncontrolled or stepped down to the lowest level that achieves sustained control.1
ICS are used first-line for maintenance treatment, starting with the lowest effective dosage and increasing to high-dose ICS as severity increases, in order to reduce airway inflammation and mucus hypersecretion.1,2 The addition of an inhaled LABA to ICS is beneficial as a patient moves up preferred treatment steps. This stimulation of beta2-receptors results in smooth-muscle relaxation followed by an increase in airflow. Alternative or adjunctive treatments include leukotriene modifiers, cromolyn, theophylline, and omalizumab.1
Eosinophilic Asthma: Treatment options for eosinophilic asthma include high-dose ICS and oral corticosteroids. Many patients with severe asthma become dependent on corticosteroids. Dependence on systemic corticosteroids for long-term maintenance therapy may harm the patient and could lead to corticosteroid resistance.10 Eosinophilic asthma presents with varying degrees of eosinophilia and elevations in immunoglobulin (Ig) E. IgE is central to the pathophysiology of allergic responses in producing early- and late-phase responses to inhaled allergens in patients with asthma.6 The monoclonal antibody (Mab) omalizumab reduces serum IgE levels, thereby reducing the response to allergens. Omalizumab is indicated for moderate-to-severe persistent asthma in patients aged 6 years with a positive skin test or in vitro reactivity to a perennial aeroallergen and symptoms that are inadequately controlled with ICS.11 This agent has been shown to reduce airway and blood eosinophils, reduce exacerbations, and decrease oral corticosteroid use in children and adults with asthma. Dosing is based on body weight and serum IgE levels. Although IgE levels do not correlate with eosinophils, anti-IgE therapy has been shown to reduce eosinophils in the airway and the blood. Patients who are unresponsive to anti-IgE therapy demonstrate persistent eosinophilic inflammation.6
New Biologics
Although anti-IgE therapy can be beneficial for eosinophilic asthma patients, IL-5 is the cytokine specific to eosinophils and an important target for therapy.6 Mepolizumab and reslizumab act upstream from IgE by binding IL-5 and reducing the signal for excess production and survival of eosinophils.12,13 See TABLE 1 for a comparison of mepolizumab and reslizumab.
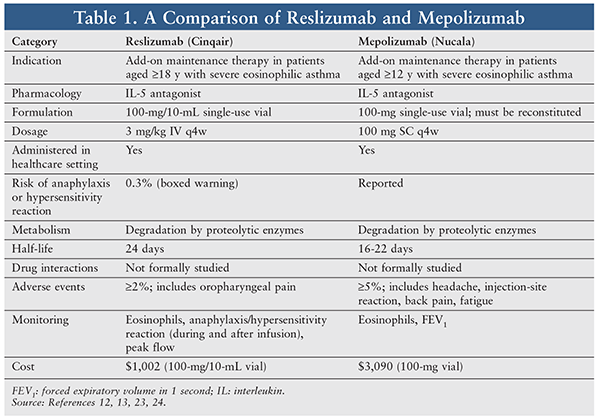
Mepolizumab (Nucala): Mepolizumab is indicated for add-on maintenance treatment of patients aged ≥12 years with severe eosinophilic asthma.12 It is a humanized IgG1 Mab degraded by proteolytic enzymes in the body and is not restricted to hepatic tissue. Mepolizumab must be reconstituted, and the recommended 100-mg dose is administered SC by a healthcare professional once every 4 weeks. The half-life ranges from 16 to 22 days. As with any maintenance medication, mepolizumab should not be used to treat acute asthma symptoms or exacerbations. The reduction of corticosteroid use following initiation of mepolizumab should be gradual and supervised by a physician. Drug interactions have not been formally studied at this time. Use during pregnancy and lactation and in patients aged <12 years or >65 years has not been sufficiently studied.12 Although the cost-effectiveness of adjunctive mepolizumab appears to exceed accepted thresholds, the use of this agent is controversial given its documented efficacy in this subset of patients with limited alternatives.14
The safety and efficacy of mepolizumab were evaluated in three double-blind, randomized trials in 1,332 patients with severe asthma associated with eosinophilic airway inflammation. These patients, aged 12 to 82 years, had a history of recurrent severe asthma exacerbations (two or more exacerbations requiring systemic corticosteroid treatment in the previous year) and signs of eosinophilic inflammation (sputum eosinophil count ≥3%, exhaled nitric oxide concentration ≥50 ppb, or peripheral blood eosinophil count ≥0.3 ´ 109/L).15-17
In the Dose Ranging Efficacy and Safety With Mepolizumab (DREAM) trial, patients were assigned (1:1:1:1) to receive 13 infusions of one of three dosages of IV mepolizumab (75 mg, 250 mg, or 750 mg) or matched placebo (0.9% NaCl 100 mL) at 4-week intervals.15 In the Mepolizumab as Adjunctive Therapy in Patients With Severe Asthma (MENSA) trial, patients were assigned to mepolizumab 75 mg IV, mepolizumab 100 mg SC, or placebo every 4 weeks for 32 weeks.16 The primary outcome of each study was the rate of clinically significant asthma exacerbations (requiring use of oral corticosteroids for ≥3 days and/or admission to an emergency department).15,16
The DREAM trial showed a significant reduction in the risk of clinically significant asthma exacerbations in mepolizumab-treated patients with severe eosinophilic asthma (1.24/year in the 75-mg group, P <.0001; 1.46/year in the 250-mg group, P = .0005; 1.15/year in the 750-mg group, P <.0001) versus patients receiving placebo (2.40/year).15 In the MENSA trial, exacerbation rates were reduced by 47% with IV mepolizumab and 53% with SC mepolizumab compared with placebo (P <.001 for both comparisons).16 Another study found that despite taking a reduced glucocorticoid dosage, the mepolizumab group had fewer asthma exacerbations (P = .04) and a lower Asthma Control Questionnaire (ACQ)–5 score (P = .004) than the placebo group.17
The safety profiles of mepolizumab and placebo in these studies were comparable. The most commonly reported adverse events were nasopharyngitis and headache.15,16 It is recommended that patients receive a varicella vaccination prior to mepolizumab therapy because herpes zoster infections have occurred in a few mepolizumab-treated patients.16
Reslizumab (Cinqair): Reslizumab is indicated for add-on maintenance treatment in patients aged ≥18 years with severe eosinophilic asthma.13 It is degraded by enzymatic proteolysis, and its half-life is approximately 24 days. Reslizumab should be administered by a healthcare professional as an IV infusion of 3 mg/kg over 20 to 50 minutes once every 4 weeks. Although drug interactions have not been formally studied, reslizumab should not be infused concomitantly with other agents. Because anaphylaxis has been observed with reslizumab, the patient must be monitored during and after infusion. Reslizumab should not be used to treat acute asthma symptoms or exacerbations. As with mepolizumab, reduction of corticosteroid use with reslizumab must be gradual and carefully monitored by a physician. Insufficient studies of reslizumab during pregnancy and lactation or in children aged <18 years have been performed. Dose reductions are not necessary in patients aged >65 years.13
The safety and efficacy of reslizumab were evaluated in four randomized phase III trials involving 4,491 patients aged 12 to 75 years with inadequately controlled, moderate-to-severe asthma and elevated blood eosinophil counts.18-20 These patients had at least one blood eosinophil count ≥400 cells/uL, had an ACQ-7 score ≥1.5 and were receiving at least a medium ICS dosage (fluticasone propionate ≥440 mcg/day or equivalent). Patients were randomly assigned to reslizumab 0.3 mg/kg IV, reslizumab 3.0 mg/kg IV, or placebo once every 4 weeks for 1 year. The primary outcome was the annual frequency of clinical asthma exacerbations per patient reported at scheduled monthly visits. Asthma exacerbations were defined as worsening of asthma that resulted in an increased use or dosage of ICS—or need for systemic corticosteroids—and a decrease in forced expiratory volume in 1 second (FEV1) of ≥20% from baseline, a reduction of ≥30% in peak expiratory flow rate, or worsening of signs or symptoms per clinician assessment.
In these studies, reslizumab significantly reduced the frequency of asthma exacerbations compared with placebo.18-20 Reslizumab 3.0 mg/kg showed better improvements in FEV1 over 16 weeks versus placebo than did reslizumab 0.3 mg/kg (P = .0018 vs. P = .0237).18 The frequency of asthma exacerbations was significantly less with reslizumab 3.0 mg/kg versus placebo (P <.0001).19 The safety profiles of reslizumab and placebo were comparable. The most common adverse events were worsening of asthma symptoms, upper respiratory tract infections, and nasopharyngitis.19,20
Anti–IL-5 Agents Currently in Development
Differing slightly from mepolizumab and reslizumab, benralizumab is an Mab that binds to the alpha chain of the IL-5 receptor to block recruitment, activation, and mobilization of eosinophils. Given this unique mechanism, benralizumab might provide a more meaningful reduction of eosinophils than mepolizumab and reslizumab. Recently completed phase III trials of benralizumab showed a reduced annual rate of asthma exacerbations, improvement in lung function, and improvement in asthma symptoms. It is anticipated that these clinical-trial data will be included in an upcoming regulatory submission for benralizumab.21,22 This agent is also being evaluated for the treatment of chronic obstructive pulmonary disease.
Role of the Pharmacist
Pharmacists need to keep abreast of current asthma guidelines and the recommended process of stepwise therapy in treating asthma patients. Given the multitude of inhalers available, pharmacists fill an essential role in teaching patients proper inhaler technique. By monitoring for excessive refill requests, the pharmacist is uniquely positioned to recognize when a patient is using asthma medications inappropriately or suffering from poorly controlled disease. The pharmacist could then speak to the patient about compliance or to the physician about the patient potentially being refractory to standard therapies. Although pharmacists may not directly dispense the new biologics—they are typically provided and administered by clinic staff—the practicing pharmacist should understand the role of these new biological agents in the treatment of severe eosinophilic asthma.
Conclusion
Asthma is a common and debilitating condition, especially for the subset of patients with severe disease that is refractory to standard treatment. Advances are being made in the treatment of eosinophilic asthma, with the newest agents, mepolizumab and reslizumab, targeting IL-5 in an attempt to reduce eosinophil-mediated inflammation. These agents, indicated for the treatment of eosinophilic asthma, are injectable monoclonal antibodies that require administration by a healthcare professional every 4 weeks. Regular use of these medications has been shown to reduce the frequency of asthma exacerbations, limit dependence on systemic corticosteroids, and reduce the impact of asthma symptoms on patients’ quality of life. As medication experts and an essential part of the healthcare team, pharmacists must be familiar with these new treatments to optimize their patients’ medication regimens.
REFERENCES
1. Global Initiative for Asthma. Global strategy for asthma management and prevention, 2016. http://ginasthma.org/wp-content/uploads/2016/04/wms-GINA-2016-main-report-final.pdf. Accessed May 26, 2017.
2. Asthma Care Quick Reference: Diagnosing and Managing Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2012. NIH Publication No. 12-5075.
3. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
4. Szefler SJ, Wenzel S, Brown R, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol. 2012;129(suppl 3):S9-S23.
5. Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033-1039.
6. de Groot JC, ten Brinke A, Bel EHD. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. 2015;1:00024-2015.
7. FDA. FDA approves Nucala to treat severe asthma. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm471031.htm. Accessed May 26, 2017.
8. FDA. FDA approves Cinqair to treat severe asthma. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491980.htm. Accessed May 26, 2017.
9. American Thoracic Society. Asthma today: ATS 2016. www.thoracic.org/patients/patient-resources/asthma-today.php. Accessed May 26, 2017.
10. Walford HH, Doherty TA. Diagnosis and management of eosinophilic asthma: a US perspective. J Asthma Allergy. 2014;7:53-65.
11. Xolair (omalizumab) package insert. South San Francisco, CA: Genentech USA, Inc; July 2016.
12. Nucala (mepolizumab) package insert. Philadelphia, PA: GlaxoSmithKline; November 2015.
13. Cinqair (reslizumab) package insert. Frazer, PA: Teva Respiratory, LLC; May 2016.
14. Whittington MD, McQueen RB, Ollendorf DA, et al. Assessing the value of mepolizumab for severe eosinophilic asthma: a cost-effectiveness analysis. Ann Allergy Asthma Immunol. 2017;118:220-225.
15. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651-659.
16. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
17. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
18. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
19. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355-366.
20. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
21. AstraZeneca. Benralizumab phase III trials show positive results in severe asthma. www.astrazeneca.com/media-centre/press-releases/2016/benralizumub-phase-III-trials-show-positive-results-in-severe-asthma- 05092016.html. Accessed May 26, 2017.
22. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
23. Reslizumab. In: AccessPharmacy Online [subscription database]. http://accesspharmacy.mhmedical.com/drugs.aspx?gbosID=238835#monoNumber=238835§ionID=146641346&tab=tab0. Accessed December 20, 2016.
24. Mepolizumab. In: AccessPharmacy Online [subscription database]. http://accesspharmacy.mhmedical.com/drugs.aspx?gbosID=238597#monoNumber=238597§ionID=147917755&tab=tab0. Accessed December 20, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.





