US Pharm. 2016;41(6):21-26.
ABSTRACT: Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by destruction of pancreatic beta-cells and loss of endogenous insulin. The treatment plan includes daily monitoring of the glucose level and an individualized insulin regimen. Multiple rapid-acting and long-acting insulin analogues are available for controlling hyperglycemia. In addition to multiple daily injections, insulin pens, insulin pumps, and inhaled insulin are alternative routes of administration. A sensor-augmented pump is an advanced technology that can benefit patients at risk of hypoglycemia. Oral administration of rapidly absorbed glucose is the antidote for treatment-related hypoglycemia, and severe cases require assistance from glucagon injection. Since T1DM pregnancies have higher risks for complications, frequent self-monitoring of blood glucose is needed. To foster normal growth and prevent long-term morbidity in adulthood, T1DM management in pediatric patients is often demanding, and parents need to establish a healthy parent-child interaction and attitude around diabetes care.
Type 1 diabetes mellitus (T1DM) is an autoimmune disease predominantly mediated by autoreactive T-cell responses that result in the destruction of pancreatic islet beta-cells and a subsequent loss of endogenous insulin.1,2 Without insulin, transport of glucose into the cells for storage is disrupted, resulting in hyperglycemia and increased serum osmolality.
Previously known as juvenile-onset diabetes, T1DM is commonly diagnosed in childhood and adolescence, occurring in about 2 per 1,000 American children.3 The rate of beta-cell destruction appears to occur more rapidly at younger ages.4 Onset also occurs in adulthood, generally before age 30 years.5 While most autoimmune diseases disproportionately affect women, T1DM appears to be more common in boys and men.6 A less common and idiopathic form of T1DM, also known as T1DM type B, shows no evidence of autoimmunity and occurs more often in individuals of African and Asian ancestry.4
CLINICAL PRESENTATION
Polydipsia (excessive thirst), poly-phagia (excessive hunger), and polyuria (excessive production of urine) are the classic trio of symptoms associated with disease onset. When kidneys eliminate the excessive glucose by using water to facilitate the glomerular filtration, a cycle of polydipsia and polyuria results until insulin is administered. In the absence of cellular glucose, fats are metabolized for energy. Ketones, the by-products of fatty-acid metabolism, subsequently accumulate and lead to ketotic breath and acidosis.
The combination of acidosis and severe dehydration associated with insulin deficiency is termed diabetic ketoacidosis (DKA).5 A life-threatening but potentially preventable condition, DKA is a common presenting symptom of newly diagnosed T1DM cases and can also be precipitated by infections, insulin pump malfunction, and any illness or procedure that can cause dehydration.7,8 DKA is more prevalent in very young patients who often have difficulty in recognizing and describing the symptoms. It is also more common in patients who are a minority, have no private health insurance, or are from a low-income household.7 Treatment of DKA includes fluid resuscitation, insulin therapy, and electrolyte replacement.8
Challenge in Diagnosis
The prevalence of T1DM is underestimated because about 5% to 15% of adult type 2 diabetes mellitus (T2DM) cases might actually be T1DM. The key distinguisher between T1DM and T2DM is the detection of autoantibodies against islet beta-cell antigens at disease presentation. About 85% to 90% of T1DM patients showed ≥1 of these autoantibodies: those reactive to insulin (IAA), glutamic acid decarboxylase 65 (GAD65), insulinoma-associated autoantigen 2 (IA2A), zinc transporter 8 (ZnT8A), and tyrosine phosphatase IA-2β and IA-2β antibodies. Accurate diagnosis of T1DM is crucial for providing proper care and preventing complications.4,6
MANAGEMENT
There is no definite cure for T1DM, and insulin therapy is necessary for life. The major goal is to maintain normoglycemia, minimize hypoglycemia, and reduce the risk of complications. A typical treatment plan includes daily monitoring of glucose levels and an individualized insulin regimen.9
Monitoring of Blood Glucose
Self-monitoring of blood glucose (SMBG) can provide guidance on proper insulin dosing and meal/snack composition. However, SMBG with a glucose meter still has gaps in following the glycemic trend to prevent hypoglycemia.10 A glucose sensor worn on the skin surface has an insertable probe that constantly monitors glucose levels in the tissue interstitial fluid. Such a real-time continuous glucose monitoring (rt-CGM) device allows patients to take immediate precautionary steps for maintaining normoglycemia. It is also useful for patients with recurrent asymptomatic hypoglycemia (i.e., hypoglycemia unawareness).9,11
In 14 randomized, controlled clinical trials, rt-CGM has led to a greater reduction in A1C and shorter durations of both hypoglycemia and hyperglycemia when compared with SMBG using a glucose meter.10 There is a lag time (up to 7 minutes) between plasma and interstitial fluid glucose, and patients need to routinely calibrate the rt-CGM device with capillary blood glucose samples for result confirmation. Accuracy of the rt-CGM device is not considered high enough by the FDA to replace the conventional glucose meters for point-of-care testing of hospitalized patients.9,11
Insulin Regimen
TABLE 1 lists the insulin analogues currently used for T1DM and their properties.9,12-17 Regimens for T1DM should include both basal and bolus prandial (meal-related) insulin as intensive insulin treatment.9 The two main approaches are:
1. Multiple daily injections (MDI) of basal insulin between meals and overnight, coupled with rapid-acting insulin or inhaled insulin before every meal
2. Continuous subcutaneous insulin infusion (CSII) to provide a more physiological way to deliver insulin.
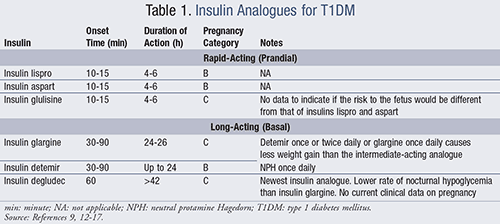
Rapid-Acting Insulin Analogues: Insulins lispro, aspart, and glulisine are created by substitution of amino acids in the B-chain of insulin. Rapid-acting analogues are practical, especially for the pediatric populations who may have variable eating habits. However, these insulins can cause intense hypoglycemia when dosed with very low carbohydrate diets.1
Long-Acting (Basal) Insulin Analogues: To increase protein binding in tissues and blood with a subsequent prolonged half-life, these analogues are created by amino acid addition or fatty-acid conjugation to the B-chain of insulin. They are the backbone of insulin therapy with longer duration of action, less peak effect, and a lower frequency of hypoglycemia.1 Insulins glargine, detemir, and degludec belong to this class and generally require only one or two injections daily.
Dosages of Insulin: The starting dose is usually weight-dependent, ranging from 0.4 to 0.5 units/kg/day of total insulin, with higher amounts required in cases of obesity, puberty, or sedentary lifestyles. Basal insulin accounts for 40% to 50% of total daily insulin. Dosage of prandial insulin is determined by the I:C ratio, which represents the units of rapid-acting insulin (I) for the amount of carbohydrates (C) in grams. This ratio can range from 1:5 (high) for insulin-resistant patients to 1:20 (low) for highly insulin-sensitive patients.9,18
Different Routes of Administration: Since many T1DM patients regard MDI as a burden, alternative routes of administration are available.
Insulin Pen: An insulin pen is equipped with a replaceable cartridge that can hold 150, 300, or 600 units of insulin and a rotatable dial for dosage selection. Pens that can deliver in half units are tailored to children and adults on low doses. Most users carry one pen of rapid mealtime insulin and another for long-acting basal insulin. Some pens contain 70/30 mixtures, which are 70% long-acting and 30% rapid-acting insulin. Compared to the vial and syringe method, insulin pens provide convenience and accuracy in delivery.1,19
CSII: Once a long-acting insulin is injected, the duration of its effect is irreversible. Therefore, to prevent the risk of hypoglycemia, patients may need snacks after a stressful event or exercise to compensate for the glucose depletion. Insulin pumps can be programmed to constantly deliver insulin, with different rates at various times, thereby offering the flexibility to accommodate the special needs associated with life activities. The pump can also offer more accurate and precise dosing, up to 1/10th to 1/20th of a unit.1
Approximately 20% to 30% of T1DM patients use CSII for a basal-bolus insulin regimen when MDI cannot provide adequate glycemic control. A basic CSII device has a dose calculator (wizard), the ability to program insulin rates, rechargeable batteries, and cartridges prefilled with basal or prandial insulin analogues.9 CSII is only recommended for highly motivated patients who are committed to insulin adjustment, carbohydrate counting, and food intake modification. Patients must be thoroughly trained and willing to maintain contact with the prescribing physician in case of pump malfunction or infection at the infusion site.9,11
A sensor-augmented pump (SAP), a combination of rt-CGM and CSII, is an advanced technology commonly indicated for patients who cannot achieve glycemic control using CSII alone or have a history of hypoglycemia unawareness. The CGM sensor is inserted at a distance from the insulin catheter, and glucose value is constantly transmitted to the insulin pump by radio frequency. When the glucose level declines below a preset threshold, the pump halts insulin delivery for 2 hours or until the patient manually restarts insulin infusion. This “threshold suspend” function is particularly helpful for patients who are at risk of hypoglycemia.9,20 SAP has been shown to be more efficient than MDI in lowering A1C for both children and adults.21
Closed-loop (CL) systems, also known as artificial pancreases, are in development. They range from fully automated insulin delivery systems requiring virtually no manual input to hybrid-CL systems requiring frequent user input, such as before mealtime or exercise.20
Inhaled Insulin: Technosphere insulin (Afrezza), approved by the FDA in 2014, provides a novel inhalation route for administering prandial insulin. Afrezza is a powdered formulation of recombinant human insulin adsorbed onto a proprietary excipient, which dissolves instantly in the lung fluid to release insulin for systemic absorption.22,23 The overall onset of action is similar to insulin lispro.24 Delivered by a dry-powder inhalation device before meals, Afrezza has shown lower incidence of hypoglycemia than the injectable insulins. Common adverse effects include cough and throat irritation. Afrezza is not recommended for smokers and is contraindicated in patients with a chronic lung disease such as asthma or chronic obstructive pulmonary disease (COPD).22,23 Spirometry testing to measure the lung capacity is required before it can be prescribed. Afrezza is classified as Pregnancy Category C, and the FDA has requested a postmarketing trial in pediatric patients.25 Unfortunately, this product has limited access, and its future is also uncertain because the manufacturer (MannKind) has recently lost its marketing partner (Sanofi) and is actively seeking a new one.26
Oral Insulin (in development): Compared to injection, oral administration is an easier route and can increase patient compliance. A high systemic insulin level from injection is associated with hypoglycemia and weight gain. In contrast, insulin taken orally can enter the circulation gradually through the hepatic portal vein to mimic endogenous insulin without the “spike.” To circumvent acidity of the stomach and the digestive enzymes along the gastrointestinal (GI) tract, the insulin has been conjugated with polyethylene glycol to create the tablet IN-105. This new oral insulin is stable, rapidly absorbed, and suitable for controlling postprandial glycemia, with no reported events of hypoglycemia and weight gain. IN-105 is currently at the late stage of clinical trials, but there is scant update on its developmental status.27
Antidote for Treatment-Related Hypoglycemia: Iatrogenic (treatment-related) hypoglycemia is the most common adverse effect of insulin therapy, occurring when blood glucose drops <70 mg/dL. It is associated with higher A1C levels, hypoglycemia unawareness, and longer disease duration. Manifestations include anxiety, behavioral changes, cognitive dysfunction, sweating, palpitations, hunger, paresthesia, tremor, seizures, and coma. Oral administration of rapidly absorbed glucose (e.g., fruit juice) is the antidote.9 Severe hypoglycemia, with blood glucose <40 mg/dL, has been linked to increased mortality.28 It requires assistance for subcutaneous (SC) or intramuscular (IM) glucagon, usually by a caregiver or a relative.9,29 Reconstitution of the glucagon powder for injection under an emergency situation can be overwhelming and highly susceptible to error.
An intranasal glucagon product, AMG504-1, is currently being investigated in clinical trials.30 This needle-free, single-use, nasal-powder dosing system is specifically designed to deliver glucagon for absorption through the nasal mucosa only without the need for deep inhalation. Therefore, a caregiver can easily administer an effective dose even to an unresponsive patient. Despite its lower bioavailability compared to SC administration, AMG504-1 has an acceptable safety profile.30
Adjunct Therapy
Pramlintide, a synthetic analogue of amylin, is an adjunct therapy to prandial insulin in T1DM. Amylin is a peptide neurohormone cosecreted with insulin by the beta-cells in response to food. It lowers postprandial glucose and enhances satiety by slowing gastric emptying, decreasing glucagon release, and promoting liver glycogen synthesis.31 As a first-in-class amylinomimetic, pramlintide complements the effects of insulin in controlling hyperglycemia, showing consistently modest A1C reduction and weight loss.9,32 Nausea is a common adverse effect, and tachyphylaxis can result after several years of use. In addition, severe hypoglycemia can occur during the initial dose adjustment period.9,31
Nondrug Support
T1DM patients should be encouraged to seek counseling from a certified diabetes educator (CDE) who is trained in diabetes management from insulin administration to foot care.9 Since cigarette smoking can increase the risk for diabetic nephropathy, retinopathy, and neuropathy, cessation of smoking is of utmost importance.33 Maintenance of normal body weight, consumption of adequate protein and heart-healthy foods, and performance of regular physical activity are also highly recommended.4,9
Vaccination
The CDC recommends vaccination against hepatitis B virus for adults (19-59 years) after a diabetes diagnosis. Despite conflicting evidence on its benefits on diabetic patients, annual influenza vaccination is also recommended for all adults.9,34,35
SPECIAL POPULATIONS
Pregnant Women
T1DM pregnancies, accounting for about 0.2% to 0.5% of pregnancies in the U.S., usually have higher risks for complications.36
Insulin sensitivity fluctuates throughout pregnancy due to changes in the hormone level. In the first trimester, increased insulin sensitivity can cause hypoglycemia, especially in women using intensive insulin regimen. Hypoglycemia symptoms such as anxiety, nausea, and tachycardia can be dangerously mistaken for pregnancy symptoms, and severe hypoglycemia can lead to unconsciousness, seizures, and even death. In contrast, increased insulin resistance during the second and third trimesters may result in hyperglycemia. Enhanced transplacental glucose transport during hyperglycemia can cause fetal hyperinsulinemia and macrosomia, a condition characterized by excessive birth weight and thus a higher risk of shoulder dystocia during delivery.5,36 Other maternal complications include preeclampsia (characterized by hypertension, proteinuria, and fluid retention), preterm delivery, and spontaneous abortion.36,37 Pregestational diabetes is also a risk factor for congenital heart defects, noncardiac birth defects, and perinatal mortality.37
Both the American Diabetes Association (ADA) and the American Congress of Obstetricians and Gynecologists (ACOG) recommend SMBG several times per day and occasionally at night. Rt-CGM devices with alarms can be beneficial to pregnant women with hypoglycemia unawareness. Insulin lispro or aspart can be used as prandial insulin, and insulin detemir as the basal insulin. All of these analogues are classified as Pregnancy Category B (TABLE 1). Despite its common use, glargine remains classified as Pregnancy Category C.9,36
Women with T1DM should receive preconception counseling, optimize A1C (<6.5%), and address medical issues before planning for a pregnancy. They also need to start folic acid supplementation (4 mg daily) and replace medications unsuitable for pregnancy.36
Young Children and Adolescents
The ADA target for A1C has been met by only 43% to 64% of pediatric patients aged <13 years and by 21% between 13 and 20 years old.38 In addition, the risk of retinopathy, nephropathy, and neuropathy is higher in children with T1DM onset at age 5 to 14 years than those diagnosed at a very young age or after puberty.39
While the treatment goal is to maintain normoglycemia, facilitation of normal growth and development should also be included in the comprehensive care. More than one-third of T1DM children are overweight or obese and have overall nutritional deficit.40 Young children with T1DM are also less physically active than their healthy peers.41 Therefore, dietary education tailored to children, with emphasis on healthy nutritional intake, coupled with a physical activity program, is critical to prevent long-term morbidity in adulthood.18 The drug treatment plan does not differ from that of adults, except that bolus prandial insulin may be given after a meal to children aged <4 years due to their inconsistent carbohydrate intake. Intermediate-acting neutral protamine Hagedorn (NPH) insulin can be used when a midday injection is difficult. Higher dosages of insulin and a higher I:C ratio for prandial insulin may be required to compensate for the insulin resistance during puberty.9 Annual screening for retinopathy and nephropathy is recommended to start between ages 9 and 11 years, depending on the disease duration,39 and routine childhood vaccinations should also be given.9
Caregivers of young children with T1DM have a higher burden of responsibility. After the initial diagnosis, a majority of young children experience a “honeymoon” phase when pancreatic beta-cell functions are temporarily restored and less insulin is needed. This period of partial remission can range from days to weeks. When it ends, level of blood glucose can increase abruptly and both caregivers and patients need to quickly adapt to the insulin regimen prescribed at diagnosis.18,42 Younger children are usually unable to comprehend and report hypoglycemia symptoms, and parents need to be vigilant about the behavioral cues that may be mistaken as temper tantrums. Without mature cognitive capabilities, these children may regard the adherence to a vigorous regimen as punitive and exhibit mealtime misbehavior and resistance to disease management. It is necessary for these parents to receive emotional support and adopt a healthy parent-child interaction around diabetes care.42
Given the adolescent need for autonomy and concerns about social context, it is very difficult for teenagers to consistently adhere to the T1DM regimen. In addition, the regimen may become more intensified to accommodate the increased insulin resistance during puberty. To increase adolescents’ motivation in managing T1DM, gratitude and positive affirmation from adults and strategies to minimize intrusion upon their daily lives are helpful.43,44
CONCLUSION
T1DM is a lifelong disease and comprehensive care is needed to maintain normoglycemia, minimize treatment-related hypoglycemia, and reduce the risk of complications. By becoming familiar with the disease and staying informed about the latest innovations of T1DM care, pharmacists can guide patients to maintain a healthy quality of life. In particular, they can explain the availability of different insulin analogues and administration routes, and how these agents can provide more accurate dosing, higher flexibility, better glycemic control, or lower hypoglycemia risk. It is important to recommend preconception care for women with T1DM who are planning for a pregnancy and dietary education and physical activity programs for pediatric patients. As very young patients with T1DM often have difficulty in recognizing and expressing discomfort, it is critical to advise their caregivers to be watchful for symptoms of hypoglycemia and DKA.
REFERENCES
1. McCall AL, Farhy LS. Treating type 1 diabetes: from strategies for insulin delivery to dual hormonal control. Minerva Endocrinol. 2013;38(2):145-163.
2. Pugliese A. Advances in the etiology and mechanisms of type 1 diabetes. Discov Med. 2014;18(98):141-150.
3. Pettitt DJ, Talton J, Dabelea D, et al. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for Diabetes in Youth study. Diabetes Care. 2014;37(2):402-408.
4. de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37(10):2843-2863.
5. Vargas R, Repke JT, Ural SH. Type 1 diabetes mellitus and pregnancy. Rev Obstet Gynecol. 2010;3(3):92-100.
6. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69-82.
7. Dabelea D, Rewers A, Stafford JM, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for Diabetes in Youth study. Pediatrics. 2014;133(4):e938-e945.
8. Gosmanov AR, Gosmanova EO, Dillard-Cannon E. Management of adult diabetic ketoacidosis. Diabetes Metab Syndr Obes. 2014;7:255-264.
9. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology—clinical practice guidelines for developing a diabetes mellitus comprehensive care plan—2015. Endocr Pract. 2015;21(suppl 1):1-87.
10. Floyd B, Chandra P, Hall S, et al. Comparative analysis of the efficacy of continuous glucose monitoring and self-monitoring of blood glucose in type 1 diabetes mellitus. J Diabetes Sci Tech. 2012;6(5):1094-1102.
11. Tumminia A, Sciacca L, Frittitta L, et al. Integrated insulin pump therapy with continuous glucose monitoring for improved adherence: technology update. Patient Prefer Adherence. 2015;9:1263-1270.
12. Tresiba (insulin degludec injection) prescribing information. Bagsvaerd, Denmark: Novo Nordisk; September 2015. www.accessdata.fda.gov/drugsatfda_docs/label/2015/203314lbl.pdf. Accessed February 28, 2016.
13. Kelley KW, Carroll DG, Meyer A. A review of current treatment strategies for gestational diabetes mellitus. Drugs Context. 2015;4:212282.
14. Russell-Jones D, Gall MA, Niemeyer M, et al. Insulin degludec results in lower rates of nocturnal hypoglycaemia and fasting plasma glucose vs. insulin glargine: a meta-analysis of seven clinical trials. Nutr Metab Cardiovasc Dis. 2015;25(10):898-905.
15. Sanlioglu AD, Altunbas HA, Balci MK, et al. Clinical utility of insulin and insulin analogs. Islets. 2013;5(2):67-78.
16. Tricco AC, Ashoor HM, Antony J, et al. Safety, effectiveness, and cost effectiveness of long acting versus intermediate acting insulin for patients with type 1 diabetes: systematic review and network meta-analysis. BMJ. 2014;349:g5459.
17. Vora J, Cariou B, Evans M, et al. Clinical use of insulin degludec. Diabetes Res Clin Pract. 2015;109(1):19-31.
18. Beck JK, Cogen FR. Outpatient management of pediatric type 1 diabetes. J Pediatr Pharmacol Ther. 2015;20(5):344-357.
19. Insulin pens. DiabetesNet. www.diabetesnet.com/about-diabetes/insulin/insulin-delivery/insulin-pens. Accessed February 28, 2016.
20. Kropff J, DeVries JH. Continuous glucose monitoring, future products, and update on worldwide artificial pancreas projects. Diabetes Technol Ther. 2016;18(suppl 2):S253-S263.
21. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320.
22. Kim ES, Plosker GL. AFREZZA (insulin human) inhalation powder: a review in diabetes mellitus. Drugs. 2015;75(14):1679-1686.
23. Ledet G, Graves RA, Bostanian LA, Mandal TK. A second-generation inhaled insulin for diabetes mellitus. Am J Health Syst Pharm. 2015;72(14):1181-1187.
24. Nuffer W, Trujillo JM, Ellis SL. Technosphere insulin (Afrezza): a new, inhaled prandial insulin. Ann Pharmacother. 2015;49(1):99-106.
25. FDA approves Afrezza to treat diabetes. FDA news release. June 27, 2014. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm403122.htm. Accessed February 26, 2016.
26. MannKind, Sanofi end licensing pact for diabetes medicine Afrezza. Wall Street Journal. January 5, 2016. www.wsj.com/articles/mannkind-sanofi-end-licensing-pact-for-diabetes-medicine-afrezza-1452008402. Accessed May 12, 2016.
27. Zijlstra E, Heinemann L, Plum-Morschel L. Oral insulin reloaded: a structured approach. J Diabetes Sci Technol. 2014;8(3):458-465.
28. McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35(9):1897-1901.
29. Frier BM. How hypoglycaemia can affect the life of a person with diabetes. Diabetes Metab Res Rev. 2008;24(2):87-92.
30. Pontiroli AE. Intranasal glucagon: a promising approach for treatment of severe hypoglycemia. J Diabetes Sci Technol. 2015;9(1):38-43.
31. Lee NJ, Norris SL, Thakurta S. Efficacy and harms of the hypoglycemic agent pramlintide in diabetes mellitus. Ann Fam Med. 2010;8(6):542-549.
32. Pullman J, Darsow T, Frias JP. Pramlintide in the management of insulin-using patients with type 2 and type 1 diabetes. Vasc Health Risk Manag. 2006;2(3):203-212.
33. Eliasson B. Cigarette smoking and diabetes. Prog Cardiovasc Dis. 2003;45(5):405-413.
34. Colquhoun AJ, Nicholson KG, Botha JL, Raymond NT. Effectiveness of influenza vaccine in reducing hospital admissions in people with diabetes. Epidemiol Infect. 1997;119(3):335-341.
35. Remschmidt C, Wichmann O, Harder T. Vaccines for the prevention of seasonal influenza in patients with diabetes: systematic review and meta-analysis. BMC Med. 2015;13:53.
36. Lenhard MJ, Kinsley BT. Insulin therapy for the treat-ment of type 1 diabetes during pregnancy. J Mater Fetal Neonatal Med. 2014;27(12):1270-1275.
37. Simeone RM, Devine OJ, Marcinkevage JA, et al. Diabetes and congenital heart defects: a systematic review, meta-analysis, and modeling project. Am J Prev Med. 2015;48(2):195-204.
38. Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035-2037.
39. Schnell O, Cappuccio F, Genovese S, et al. Type 1 diabetes and cardiovascular disease. Cardiovasc Diabetol. 2013;12:156.
40. Mehta SN, Volkening LK, Quinn N, Laffel LM. Intensively managed young children with type 1 diabetes consume high-fat, low-fiber diets similar to age-matched controls. Nutr Res. 2014;34(5):428-435.
41. Sundberg F, Forsander G, Fasth A, Ekelund U. Children younger than 7 years with type 1 diabetes are less physically active than healthy controls. Acta Paediatrica. 2012;101(11):1164-1169.
42. Streisand R, Monaghan M. Young children with type 1 diabetes: challenges, research, and future directions. Curr Diab Rep. 2014;14(9):520.
43. Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr. 2010;22(4):405-411.
44. Jaser SS, Patel N, Linsky R, Whittemore R. Development of a positive psychology intervention to improve adherence in adolescents with type 1 diabetes. J Pediatr Health Care. 2014;28(6):478-485.
To comment on this article, contact rdavidson@uspharmacist.com.






