ABSTRACT: The coronavirus 2019 pandemic has caused dramatic changes in daily life, primarily in terms of social connections. Many people are using videoconferencing via Zoom and similar programs as a means of communication for work, school, and social events. As a result, Zoom dysmorphia—which can potentially cause or worsen body dysmorphic disorder (BDD)—is a growing concern. Owing to the lack of FDA-approved agents for BDD, treatment involves psychosocial and pharmacologic therapies that target the underlying issues. It is important to identify underlying BDD in Zoom-dysmorphic patients and to use published guidelines, multimodal treatment strategies, and individual patient response to determine the best approach for effective BDD management.
Videoconferencing via Zoom, Google Hangouts, Microsoft Teams, and similar programs (henceforth collectively referred to as “Zoom”) has become the primary means of communication for work, school, and social events during the coronavirus 2019 (COVID-19) pandemic. Prior to the advent of Zoom, individuals could edit or enhance still digital images of themselves by making cosmetic changes (e.g., removing acne, adding makeup, altering the nose) to create a filtered image that was more acceptable to them. Consequently, esthetic professionals began to receive requests from patients who wanted to look identical to their filtered image. This phenomenon, known as Snapchat dysmorphia, caused worldwide concern for its potential to precipitate or worsen body dysmorphic disorder (BDD), which is characterized by excessive preoccupation with an imagined or minor physical imperfection.1 Unlike Snapchat, Zoom shows images in real time and provides minimal ability to enhance or alter the video image that is visible to others.2 In Zoom dysmorphia, continued dissatisfaction with one’s personal appearance combined with prolonged exposure to the perceived unsettling image may have an even greater potential to trigger or worsen BDD, making this recent phenomenon a growing concern.2
Although both Zoom dysmorphia and BDD may lead someone to seek professional cosmetic enhancements, there is a distinct difference between dislike of the specific image visible on Zoom and the persistent dissatisfaction with one’s physical appearance, even outside of Zoom, that occurs with BDD.1 It is estimated that nearly 9.2% of cosmetic outpatients and 11.3% of dermatology outpatients have BDD.3 Frequently, cosmetic surgery has caused BDD patients to develop new appearance concerns or become agitated or dissatisfied over minor imperfections or the results of the procedure, sometimes leading to threats of violence or legal action against the surgeon.4,5 Cosmetic surgery is not recommended as an appropriate treatment for BDD patients, as enhancements fail to resolve dissatisfaction and may worsen BDD severity.4 It is instead recommended that therapy targeted at resolving the underlying issues be implemented in these patients.4
Overview of BDD
BDD, which is complex and often severe, affects nearly 3% of the U.S. population.6 Data suggest that BDD is more common in women than in men (2.5% vs. 2.2%) and is typically more prevalent among college students (5%).6-8 People with BDD believe that they have a noticeable physical abnormality, whereas the perceived flaw is actually indiscernible to others.9 The skin (usually facial) is usually the area of dissatisfaction.10
BDD is often misdiagnosed as one of its commonly comorbid conditions: major depressive disorder, obsessive-compulsive disorder (OCD), or social anxiety.10 Because the symptoms of BDD and these comorbid disorders may be similar, it is important to thoroughly interview the patient in order to make an accurate diagnosis, and if BDD is suspected, this should be specifically addressed with the patient. BDD is typically associated with past trauma, and these patients may demonstrate low self-esteem, depressive symptoms, anxiety, and heightened rejection sensitivity. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, classifies BDD under OCD, largely because of the compulsive behaviors performed in response to physical concerns.11 These behaviors may include continued grooming of perceived defects, reassurance-seeking, constant mirror-checking, and marked avoidance of social situations.9,11 The following criteria must be met for clinical diagnosis of BDD:
1. The patient has a preoccupation with one or more perceived deficits or flaws in physical appearance that are not observable or are slight to others.
2. The patient performs repetitive behaviors or mental acts (e.g., comparing one’s appearance to that of others) in response to the appearance concerns at some point during the disorder.
3. The preoccupation causes clinically significant distress or impairment in social, occupational, or other areas of functioning.
4. The preoccupation is not better explained by concerns meeting diagnostic criteria for eating disorders.11
BDD is believed to be due to a combination of biological, environmental, and psychological factors.9 In patients with BDD, neuroimaging reveals abnormal structure and function of occipitotemporal and frontolimbic regions as well as reduced dopamine D2/D3 receptor availability, suggesting pathophysiology related to dopamine pathways that enables potential drug targets.12,13 Various targeted treatments are available to patients diagnosed with BDD.
BDD Treatment
Treatment of BDD should be targeted to the underlying psychosocial issues. Additionally, prior to therapy initiation, motivational interviewing may be undertaken to build a rapport with the patient in order to foster his or her engagement and adherence.10 Although research regarding BDD treatment is limited and no FDA-approved agents specifically target BDD, the therapies listed in TABLE 1 have been proven effective.9,14 According to the 2005 National Institute for Health and Care Excellence guidelines, cognitive-behavioral therapy (CBT) directly addressing BDD is the psychosocial treatment of choice. CBT follows a protocol over 16 to 24 sessions that aims to help the patient gain a deeper understanding of his or her difficulties while reducing self-defeating attention practices.14 Although CBT is efficacious, many researchers have noted that the accessibility of pharmacologic therapies may make them a more viable option.15
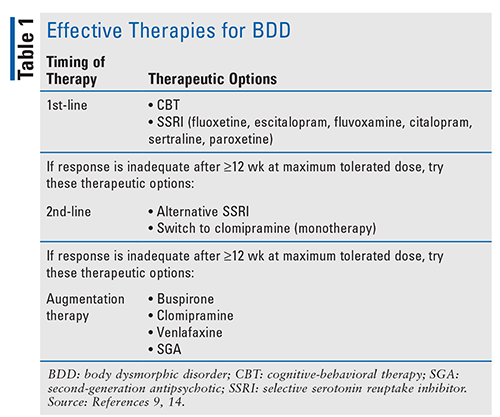
Results from the limited randomized, controlled trials on BDD therapy support selective serotonin reuptake inhibitors (SSRIs) as the first-line pharmacologic option.14 No agent is superior among SSRIs, but fluoxetine and escitalopram are used most often.9 Fluoxetine use is based on results of a study by Phillips and colleagues in which fluoxetine was significantly more effective than placebo.16 Historically, SSRIs have been the best-tolerated antidepressant class, given their superior safety and adverse-effect (AE) profile (TABLE 2).17-19 Caution should be exercised with citalopram use because of the cardiac effects seen with the higher doses required for BDD symptom improvement.9 Citalopram has a dosing limit of 40 mg per day owing to increased risk of QTc prolongation at higher doses, possibly making it a less favorable option.20
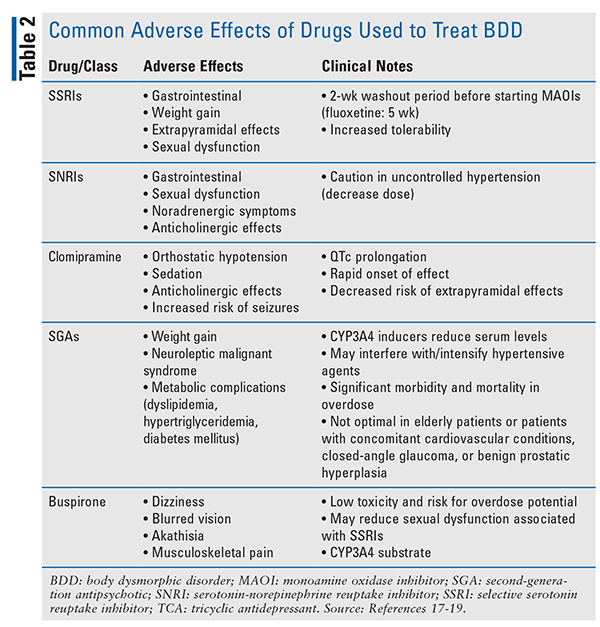
The tricyclic antidepressant (TCA) clomipramine has demonstrated superiority over desipramine in relieving BDD symptoms, depressive symptoms, and functional disability, which has led to its implementation as a second-line option for monotherapy or augmentation therapy.21 Typically, clomipramine is reserved for cases in which the initial SSRI and alternatives have been ineffective.9 Caution should be exercised given the increased risk of intentional overdose with TCAs, as BDD is associated with significantly higher suicide-completion rates compared with other mental disorders.16,17 Additionally, the dosage of clomipramine should not exceed 250 mg per day.22 For BDD patients who are at greater risk for suicide, an SSRI should be considered, and its use in combination with CBT is recommended.10
Five open-label trials demonstrated improvements in both BDD and associated symptoms in approximately 60% to 85% of patients using fluvoxamine, citalopram, escitalopram, or sertraline.3 Data have also revealed benefits from the initiation of buspirone, venlafaxine, or antipsychotics for augmentation therapy in patients who have refractory symptoms with the aforementioned therapy or are experiencing BDD symptoms in conjunction with increased agitation, anxiety, or delusions.21 Typically, augmentation therapy is selected based on common AEs and pharmacologic properties (TABLE 2) or comorbid conditions for which certain agents may be more beneficial.17
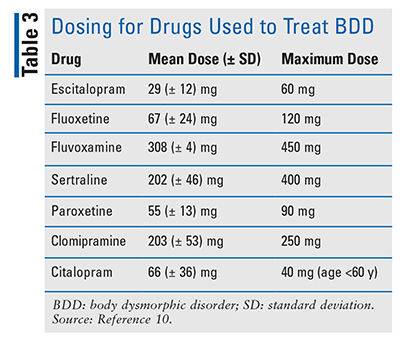
Data on appropriate dosing for pharmacologic therapy for BDD are limited. Research has shown that symptom improvement may require dosing similar to that for OCD but much higher than that typically used for other disorders.21 When an SSRI is ineffective, improvement may occur when the dose is increased, in some cases beyond the maximum recommended by the manufacturer.10 Some researchers have studied dosages as high as 400 mg per day for fluvoxamine and 80 mg to 100 mg per day for citalopram or paroxetine.10,21 An article by Phillips included data on mean and maximum daily dosing used in BDD patients, with dosages ranging from 29 mg per day to 450 mg per day (TABLE 3); this information further illustrates the elevated dosing requirements for this disease state.10
In most studies, 6 to 9 weeks was the average time needed for BDD patients to respond to therapy; however, some patients required 12 to 14 weeks.23 Patients should receive therapy for a minimum of 12 weeks, with at least 3 to 4 of those weeks at maximum tolerated doses before changes are made.9,10
Relapse and Refractory Disease
Roughly 20% of patients will adequately recover from BDD, but 40% will subsequently relapse, typically related to earlier age of onset and severity of BDD symptoms at presentation.9 In an effort to prevent relapse following CBT, patients prepare to terminate formal treatment and continue implementing learned strategies for diminishing the compulsive behaviors.10 Likewise, continuous maintenance therapy has been shown to extend the duration of remission or time to relapse in most patients.9 Escitalopram is considered an appropriate choice for relapse prevention in BDD based on study findings that times to relapse were longer and relapse rates were less in patients receiving continuous therapy.24 Additionally, antidepressants—particularly those with a shorter half-life (e.g., paroxetine)—generally should not be discontinued abruptly following extended therapy given the risk of rebound anxiety or discontinuation syndrome.17
Patients are deemed refractory to BDD treatment after failing two adequate trials of at least two antidepressant classes. Refractory BDD is most commonly a result of inadequate dosage or trial length for SSRIs, lack of patient compliance, or failure to augment SSRIs. Therefore, it is important to optimize dosing, titration, and augmentation protocols so that the patient receives maximum benefit. In refractory patients, combined pharmacologic and CBT therapies should be recommended, and partial hospitalization or residential treatment may be required.10
COVID-19 Pandemic and BDD
The COVID-19 pandemic has created many difficulties for the general population, but even more problems are experienced in persons diagnosed with mental-health disorders, including heightened symptom severity and psychosocial stress.25 In one survey, 33.7% of participants with mental-health disorders (including BDD) stated that their symptoms were slightly worsened because of the COVID-19 pandemic.25 The sudden shutdown of beauty services during the pandemic led to AEs in BDD patients who used these services as a coping mechanism, according to another survey.26 A different survey found that 36.6% of respondents with BDD were willing to leave home to undergo plastic surgery during the pandemic despite awareness of the potential risks, versus 6.6% of respondents without BDD.27
In studying the ongoing virtual communication necessitated by the pandemic, researchers have discovered that continuous Zoom use prompts constant distress in persons with body dissatisfaction. Individuals who spent more time on Zoom have reported decreased face and body satisfaction.2 Additionally, the more appearance comparisons an individual engaged in on Zoom, the more body-image concerns and facial dissatisfaction he or she had.2
Conclusion
The COVID-19 pandemic has caused a societal shift that requires the regular use of videoconferencing programs such as Zoom to maintain communication. Zoom dysmorphia has raised concern because of its potential to trigger or worsen BDD. Therefore, it is important to identify the presence of underlying BDD in Zoom-dysmorphic patients and treat it appropriately. The goal is to accurately diagnose and treat BDD patients while emphasizing the avoidance of cosmetic procedures. Pharmacists can play an integral role in the management of BDD by carefully monitoring for drug interactions that would diminish the effectiveness of an antidepressant and by effectively counseling patients on possible AEs and the importance of adherence when these medications are used.
REFERENCES
1. Rajanala S, Maymone MBC, Vashi NA. Selfies—living in the era of filtered photographs. JAMA Facial Plast Surg. 2018;20(6):443-444.
2. Pfund GN, Hill PL, Harriger J. Video chatting and appearance satisfaction during COVID-19: appearance comparisons and self-objectification as moderators. Int J Eat Disord. 2020;53(12):2038-2043.
3. Singh AR, Veale D. Understanding and treating body dysmorphic disorder. Indian J Psychiatry. 2019;61(suppl 1):S131-S135.
4. Phillips KA, Grant J, Siniscalchi J, Albertini RS. Surgical and nonpsychiatric medical treatment of patients with body dysmorphic disorder. Psychosomatics. 2001;42(6):504-510.
5. Sarwer DB. Awareness and identification of body dysmorphic disorder by aesthetic surgeons: results of a survey of American Society for Aesthetic Plastic Surgery members. Aesthet Surg J. 2002;22(6):531-535.
6. International OCD Foundation. About BDD for professionals. https://bdd.iocdf.org/professionals/. Accessed April 13, 2021.
7. Koran LM, Abujaoude E, Large MD, Serpe RT. The prevalence of body dysmorphic disorder in the United States adult population. CNS Spectr. 2008;13(4):316-322.
8. Bohne A, Wilhelm S, Keuthen NJ, et al. Prevalence of body dysmorphic disorder in a German college student sample. Psychiatry Res. 2002;109(1):101-104.
9. Hong K, Nezgovorova V, Uzunova G, et al. Pharmacological treatment of body dysmorphic disorder. Curr Neuropharmacol. 2019;17(8):697-702.
10. Phillips K. Body dysmorphic disorder: clinical aspects and relationship to obsessive-compulsive disorder. Focus. 2015;13(2):162-174.
11. Substance Abuse and Mental Health Services Administration. DSM-5 changes: implications for child serious emotional disturbance (2016). Table 23, DSM-IV to DSM-5 body dysmorphic disorder comparison. www.ncbi.nlm.nih.gov/books/NBK519712/table/ch3.t19/. Accessed April 9, 2021.
12. Grace SA, Buchanan BG, Maller JJ, et al. Reduced cortical thickness in body dysmorphic disorder. Psychiatry Res Neuroimaging. 2017;259:25-28.
13. Vulink NC, Planting RS, Figee M, et al. Reduced striatal dopamine D2/3 receptor availability in body dysmorphic disorder. Eur Neuropsychopharmacol. 2016;26(2):350-356.
14. National Institute for Health and Care Excellence. Obsessive-compulsive disorder and body dysmorphic disorder: treatment. www.nice.org.uk/guidance/cg31. Accessed January 26, 2021.
15. Krebs G, Fernández de la Cruz L, Mataix-Cols D. Recent advances in understanding and managing body dysmorphic disorder. Evid Based Ment Health. 2017;20(3):71-75.
16. Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch Gen Psychiatry. 2002;59(4):381-388.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Association; 2013.
18. Wilson TK, Tripp J. Buspirone. In: StatPearls. Treasure Island, FL: StatPearls Publishing; January 2021. www.ncbi.nlm.nih.gov/books/NBK531477/. Accessed April 9, 2021.
19. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
20. FDA. Clarification of dosing and warning recommendations for Celexa. www.fda.gov/drugs/special-features/clarification-dosing-and-warning-recommendations-celexa. Accessed February 18, 2021.
21. Phillips KA. Body dysmorphic disorder: recognizing and treating imagined ugliness. World Psychiatry. 2004;3(1):12-17.
22. Wilson M, Tripp J. Clomipramine. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; January 2021. www.ncbi.nlm.nih.gov/books/NBK541006/. Accessed January 26, 2021.
23. Phillips KA. Pharmacologic treatment of body dysmorphic disorder: review of the evidence and a recommended treatment approach. CNS Spectr. 2002;7(6):453-460.
24. Phillips KA, Keshaviah A, Dougherty DD, et al. Pharmacotherapy relapse prevention in body dysmorphic disorder: a double-blind, placebo-controlled trial. Am J Psychiatry. 2016;173(9):887-895.
25. Quittkat HL, Düsing R, Holtmann FJ, et al. Perceived impact of Covid-19 across different mental disorders: a study on disorder-specific symptoms, psychosocial stress and behavior. Front Psychol. 2020;11:586246.
26. Pikoos TD, Buzwell S, Sharp G, Rossell SL. The COVID-19 pandemic: psychological and behavioral responses to the shutdown of the beauty industry. Int J Eat Disord. 2020;53(12):1993-2002.
27. Gelidan AG, Mrad MA, Kattan AE, et al. The public’s awareness and willingness to undergo plastic surgery procedures during the COVID-19 pandemic. Plast Reconstr Surg Glob Open. 2020;8(9):e3170.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






