US Pharm. 2016;41(6):42-45.
ABSTRACT: Kawasaki disease is a febrile systemic vasculitis that primarily affects the coronary arteries. More than 80% of patients are aged 6 months to 5 years, and the prevalence is especially high in the Japanese population. A number of theories, including those based on autoimmunity and genetics, have been proposed to explain the underlying cause of the condition, but the etiology remains debatable. The clinical symptoms of Kawasaki disease are well defined, particularly a fever of ³101°F, but there are no differential diagnostic criteria. Primary therapy includes administration of IV immunoglobulin and aspirin for the management of the acute phase. In patients who develop coronary disease, long-term management for the prevention and treatment of thrombosis includes antiplatelet, anticoagulant, and thrombolytic agents.
Kawasaki disease is a febrile systemic vasculitis of childhood that primarily affects the coronary arteries. It is the most common cause of acquired heart disease in children in the United Kingdom and the United States, and it may be a risk factor for ischemic heart disease in adults.1,2 Kawasaki disease was first identified in Japan in 1967 by Tomisaku Kawasaki, and its first appearance in English-language medical literature was in 1974.3,4
Epidemiology
Although Kawasaki disease occurs worldwide, it is overreported among persons of Asian ethnicity, especially the Japanese population. The prevalence is 67 cases per 100,000 children in Japan, compared with 5.6 per 100,000 in the U.S.4-6 Kawasaki disease primarily affects children aged 6 months to 5 years, and 80% of patients are aged <5 years.7-9 The disorder is rare in neonates and adolescents, and it is more common in males than in females (ratio of 5:1).9
Etiology
Although the clinical symptoms of Kawasaki disease are well characterized, the etiology has not yet been established. Kawasaki disease mirrors toxin-mediated disease from both a clinical and an immunologic perspective.9 A number of theories regarding the underlying cause of Kawasaki disease have been proposed, including autoimmunity and genetic factors. Since the prevalence of Kawasaki disease is highest in children aged 6 months to 5 years—when most children stop breastfeeding and maternal immune function decreases—it is possible that an underlying infectious disease is responsible. Infectious agents including Propionibacterium acnes, Rickettsia, Epstein-Barr virus, and retroviruses may be involved, but this has yet to be confirmed.8,10-12
Proinflammatory cytokines also are believed to play a role in disease pathogenesis.13 This theory suggests that Kawasaki disease may be caused by an immune-mediated response in certain individuals who are genetically or immunologically susceptible to a powerful immunostimulant product, the superantigen of an infectious agent. There is strong evidence for both theories, and an increased frequency of alleles associated with elevated tumor necrosis factor-alpha (TNF-alpha) levels has been found.14,15
Patients with Kawasaki disease generally recover well, and the condition is self-limiting in most cases.8 However, Kawasaki disease can lead to devastating cardiovascular (CV) complications.
Clinical Manifestations
Kawasaki disease carries great clinical importance and must be diagnosed and managed appropriately.8 No diagnostic test for Kawasaki disease is available to date, and analysis is based on clinical criteria after the exclusion of certain diseases that present with high, persistent fever.16 Since prompt treatment with immunoglobulins and aspirin has been shown to reduce the rate of coronary abnormalities to <5% of patients, early diagnosis is critical.9
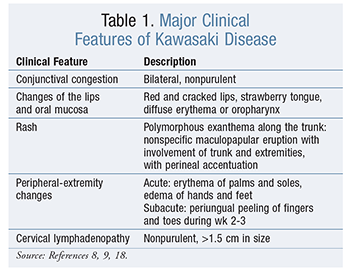
The course of Kawasaki disease may be divided into three clinical phases: acute febrile, subacute, and convalescent. The acute febrile phase usually lasts 1 to 2 weeks, during which time the fever is often high, spiking, and persistent or recurrent despite the administration of antibiotics or antipyretics. The classic diagnostic criteria include fever (>101°F) of >5 days’ duration plus four of the five major clinical features: conjunctival congestion, cervical lymphadenopathy, oral mucosal changes, polymorphous rash, and swelling and redness of the extremities (TABLE 1).8,9,17,18 Alternatively, fever plus coronary artery aneurysms (CAA) detected via two-dimensional echocardiography (2D-ECHO) and the presence of three clinical features also signify Kawasaki disease.9
Other findings include lethargy and irritability (possibly due to aseptic meningitis), vomiting, diarrhea, cough, rhinorrhea, acute surgical symptoms, hydrops of the gallbladder, arthritis and arthralgia affecting the larger joints in particular, and uveitis.9 Since uveitis can lend additional support to the diagnosis in patients with incomplete Kawasaki disease, an ocular evaluation should be included in the workup for any patient suspected to have Kawasaki disease.9 Erythema also may be seen on bacillus Calmette-Guérin vaccination sites in children aged <2 years.19
During the acute phase, endothelial cells swell and undergo necrosis, and the intima and vasa vasorum of the larger arteries are inflamed. The subacute phase, which lasts approximately 10 to 25 days after the onset of fever, is associated with inflammation of the coronary arteries that leads to the formation of aneurysms.20 The convalescent phase usually lasts 6 to 8 weeks, starting once the clinical presentations of Kawasaki disease abate and continuing until the erythrocyte sedimentation rate (ESR) normalizes.18 During this phase, the acute inflammation subsides.20
Incomplete Kawasaki Disease
In incomplete (atypical) Kawasaki disease, the patient has persistent fever, but possesses either fewer than four of the five diagnostic criteria or three of the five diagnostic criteria plus CAA detected via 2D-ECHO. Since incomplete Kawasaki disease is most common in young infants, who are at greatest risk for developing CAA, it is important that the patient be thoroughly tested.21
There are no specific laboratory findings for Kawasaki disease, and many of the results of common laboratory tests may be shared by other acute inflammatory febrile diseases. In the early phase, ESR, C-reactive protein (CRP), and WBC and neutrophil counts may be increased. Since ESR is not a reliable indicator once IV immunoglobulin (IVIG) has been administered, CRP should be used to assess improvement as a marker of inflammation. In the acute phase, the platelet count is normal (or, in rare cases, low) and markedly increases after about 14 days. Other findings may include anemia, abnormal plasma lipids, hypoalbuminemia, hyponatremia, thrombocytosis after 7 days, sterile pyuria, elevated serum transaminases, elevated serum gamma glutamyl transpeptidase, pleocytosis of cerebrospinal fluid, and leukocytosis in synovial fluid.9,18
Cardiac Investigations
CV complications such as coronary artery lesions can lead to long-term morbidity and mortality in Kawasaki disease; therefore, ECG and 2D-ECHO monitoring for cardiac complications form a vital part of disease management.22,23 Without timely treatment during the acute phase, about 20% of patients develop CAA.8 Giant aneurysms (diameter >8 mm) develop in approximately 0.5% to 1% of patients; once such an aneurysm forms, recovery is almost impossible, and coronary stenosis, obstruction, or acute thrombosis may result. These complications cause ischemic heart disease and/or myocardial infarction (MI) eventually and may also cause rare but fatal sudden cardiac death in children.24,25 MI, in particular, has a poor prognosis, with a reported mortality of 22% after the first MI and 70% to 80% mortality after subsequent episodes.26
Peak mortality from coronary vasculitis and aneurysms occurs 15 to 45 days after the onset of fever; however, there have been sudden deaths from MI that occurred months to many years after Kawasaki disease and were attributed to untreated Kawasaki disease in childhood.27
Treatment
The goals of therapy in the management of Kawasaki disease are to prevent coronary artery disease and to relieve symptoms. The first-line standard therapy for patients with confirmed or suspected Kawasaki disease is a combination of IVIG and a salicylate, typically aspirin.28 It has been shown that the prevalence of coronary artery abnormalities in patients with Kawasaki disease depends on the dose of immunoglobulin, but not that of aspirin.29,30
The role of IVIG in reducing coronary artery abnormalities is well established, but the mode of action is unknown.29-32 IVIG, which relieves acute inflammation, should be administered ideally as soon as the disease is suspected and possibly within the first 10 days of the onset of fever. Although clinical data supporting the use of IVIG >10 days after onset are lacking, IVIG is administered past the 10-day cutoff if the patient demonstrates persistent signs of inflammation.9,18
The optimal dosage of IVIG is 2 g/kg given as a single infusion over 10 to 12 hours.33 This regimen, administered together with aspirin within the first 10 days, leads to resolution of clinical symptoms in 80% to 90% of patients, and the reduction in risk of coronary disease ranges from 20%-25% to 2%-4%.34 Some patients may not tolerate the fluid challenge associated with a single infusion; therefore, a divided dose over several days may be appropriate for infants with cardiac compromise. Some clinicians prescribe IVIG administration at a rate of at 0.025 g/kg/hour for 15 minutes, doubling the rate every 15 minutes until a rate of 0.2 g/kg/hour is reached, and then every 30 minutes for 1 hour and finally every hour until the infusion is complete, with vital signs monitored before every change.18
Adverse effects associated with IVIG seem to vary greatly between brands, possibly because of the potential manufacturing differences.35 Administration of live-attenuated vaccines, including those for measles and varicella, should be delayed until 9 to 11 months after receipt of IVIG. Other childhood vaccinations should not be delayed or withheld.35 If the risk of exposure to measles is high, the child may be immunized earlier and then reimmunized within 11 months after IVIG administration.
Aspirin has both anti-inflammatory and antiplatelet activity, and it works in synergy with IVIG. Even though it has been the mainstay of treatment for many decades, no prospective study has confirmed that aspirin reduces the incidence of coronary artery abnormalities.18 During the acute phase, aspirin is given at anti-inflammatory doses of 80 to 100 mg/kg either until the patient has been afebrile for 48 hours or for 14 days.35 The dosage is then reduced to 3 to 5 mg/kg, and in the absence of cardiac complications, low-dose aspirin is maintained until the ESR and platelet count are normal. If coronary artery abnormalities are detected, however, long-term low-dose aspirin is indicated to prevent thrombosis.9,18 Lifelong aspirin therapy is indicated in a child if aneurysms persist.35
Children on long-term aspirin therapy are at risk for developing Reye syndrome, especially if they experience an active infection with varicella or influenza; therefore, they should receive an annual influenza vaccine.35-37 Furthermore, parents of children on long-term aspirin therapy should be advised to contact their physician immediately if the child shows symptoms of, or is exposed to, influenza or varicella. It is recommended that salicylates be avoided for 6 weeks after the administration of varicella vaccine. Physicians, however, should evaluate the risk of varicella vaccine against the known risk of varicella in children receiving long-term salicylate therapy.35 In some cases, an alternative antiplatelet medication such as dipyridamole may be substituted during this 6-week period.35 Since ibuprofen antagonizes the effects of aspirin, it should not be given to children with coronary aneurysms who are taking aspirin.35
Pentoxifylline is a methyl xanthine compound that specifically inhibits TNF-alpha messenger RNA transcription. It has anti-inflammatory activity, and in Kawasaki disease it may reduce the incidence of aneurysms by blocking TNF-alpha in the inflammatory cascade.35,38 Pentoxifylline has been used in some patients with Kawasaki disease, although it is not an established first-line therapy.35
Nonresponders to Initial Therapy
In most children with Kawasaki disease, the fever will abate during or a few hours following an IVIG infusion. However, a small number of patients—10% to 15%—fail to respond to an initial single dose of IVIG and oral aspirin.39,40 Some patients may respond initially, but become febrile again after a short period.
In patients who do not respond to the initial IVIG infusion and present with persistent fever or disease recrudescence, a second or even a third cycle may be required.35,39,41 Most experts administer a second dose of IVIG, as recommended by the American Heart Association (AHA) consensus guidelines.35 It should be noted that even though there are no controlled studies demonstrating its effectiveness, a second dose of IVIG appears to be effective in most patients.39,41,42 Patients who fail to respond to two doses of IVIG present a unique challenge, as the appropriate treatment remains uncertain. Most Kawasaki disease experts will consider a third dose of IVIG, infliximab, or a trial of methylprednisolone.18
Corticosteroids
The use of corticosteroids is recommended in patients who fail to respond to initial therapy for Kawasaki disease.35,43 Pulse methylprednisolone, given at supraphysiological doses of 30 mg/kg daily IV for 1 to 3 days, has been reported in a case series and one small controlled trial and is the most commonly used regimen.35,43 The AHA recommends restriction of pulse corticosteroids to children in whom two or more IVIG infusions have been ineffective.35
Other therapeutic agents that have been used for nonresponders include the following:
• Plasma exchange: Even though plasma exchange has been shown to be effective in children who are refractory to IVIG therapy, it is not generally recommended because of its risks.35
• Ulinastatin: Ulinastatin, a human trypsin inhibitor extracted from human urine, has demonstrated some effectiveness in IVIG-refractory Kawasaki disease patients in Japan. It is believed to function by inhibiting neutrophil elastase and prostaglandin H2 synthase at the messenger RNA level, but there is not enough evidence to support its use.38
• Abciximab: Abciximab is a platelet glycoprotein IIb/IIIa receptor inhibitor that has been used in conjunction with standard therapies in patients with Kawasaki disease and giant aneurysms.35,38
• Monoclonal antibodies: Infliximab is a chimeric human-mouse monoclonal antibody that specifically binds to human TNF-alpha. Although studies have yet to demonstrate its effectiveness in reducing the prevalence of CAAs, infliximab may be useful in patients who are resistant to first-line therapy.35,38 One study comparing a second IVIG infusion (2 g/kg) with infliximab (5 mg/kg) after initial treatment with IVIG found that infliximab was as effective as the second dose of IVIG.44
• Cyclosporine: Given the role of activated T cells in the pathogenesis of vasculitis in patients with Kawasaki disease, T-cell inhibitors like cyclosporine have been suggested as a treatment option for refractory Kawasaki disease.18
• Methotrexate (MTX): The anti-inflammatory properties of low-dose MTX have led to its use in the treatment of autoimmune diseases, including rheumatoid arthritis. Although the exact mechanism by which low-dose MTX modulates inflammation is unknown, it is likely that different levels of the pathophysiological cascade are affected, similar to those in Kawasaki disease.18
• Cytotoxic agents: Cyclophosphamide with or without MTX or corticosteroids has been suggested as possible therapy for IVIG-refractory patients with Kawasaki disease.40
Thrombosis Prevention and Treatment in Patients With Coronary Disease
Possible regimens for the prevention of thrombosis in patients with Kawasaki disease include antiplatelet and/or anticoagulant therapy. The regimen and dosing vary based on the severity of coronary involvement and may include orally administered low-dose aspirin, dipyridamole, clopidogrel, warfarin, or low-molecular-weight heparin. Dipyridamole 2 to 6 mg/kg per day in three divided doses is a useful alternative in children with aspirin intolerance.9 Giant aneurysms are typically managed with aspirin and warfarin 0.1 mg/kg per day, adjusted to an international normalized ratio of 2.0 to 2.5.18
Thrombus initiation in Kawasaki disease leads to a thrombus burden unlike that seen in adult atherosclerotic coronary occlusion; therefore, thrombolytic regimens that are effective in adults with atherosclerotic coronary disease may not necessarily be useful in children with Kawasaki disease. Thrombolytic agents such as streptokinase, urokinase, and tissue plasminogen activator have been tested in children with coronary thrombosis.35
Surgical management of Kawasaki disease consists primarily of coronary artery bypass grafts for obstructive lesions. Catheter interventional treatments include balloon angioplasty, rotational ablation, and stent placement.35
Prognosis
Even with prompt IVIG treatment, coronary disease is not uncommon in Kawasaki disease, and morbidity remains high.1 One study found cardiac sequelae due to Kawasaki disease in 10.2% of patients 1 month after onset and in 4.2% after 1 year.45 Those at highest risk for coronary artery disease are infants aged <6 months and older children with an extremely high platelet count, high ESR, and fever lasting >2 weeks.9
Conclusion
Although Kawasaki disease was identified more than 50 years ago, its etiology remains to be clarified. Patients who develop the disease are at risk for being underrecognized or misdiagnosed with other febrile illnesses.9 Recent advances in Kawasaki disease research include searches for genetic susceptibility related to the condition and investigation into immunopathogenesis based on the idea that innate immunity plays a more prominent role than the acquired immunity.8
REFERENCES
1. Nakamura Y, Yanagawa H, Harada K, et al. Mortality among persons with a history of Kawasaki disease in Japan: existence of cardiac sequelae elevated the mortality. J Epidemiol. 2000;10:372-375.
2. Silva AA, Maeno Y, Hashmi A, et al. Cardiovascular risk factors after Kawasaki disease: a case-control study. J Pediatr. 2001;138:400-405.
3. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children [article in Japanese]. Arerugi. 1967;16:178-222.
4. Kawasaki T, Kosaki F, Okawa S, et al. A new infantile febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271-276.
5. Taubert KA. Epidemiology of Kawasaki disease in the United States and worldwide. Prog Pediatr Cardiol. 1997;6:181-185.
6. Yanagawa H, Kawasaki T, Shigematsu I. Nationwide survey on Kawasaki disease in Japan. Pediatrics. 1987;80:58-62.
7. Rauch AM. Kawasaki syndrome: critical review of U.S. epidemiology. Prog Clin Biol Res. 1987;250:33-44.
8. Kim KY, Kim DS. Recent advances in Kawasaki disease. Yonsei Med J. 2016;57:15-21.
9. Cimaz R, Falcini F. An update on Kawasaki disease. Autoimmun Rev. 2003;2:258-263.
10. Leung DY. Kawasaki syndrome: immunomodulatory benefit and potential toxin neutralization by intravenous immune globulin. Clin Exp Immunol. 1996;104(suppl 1):49-54.
11. Leung DY, Meissner HC, Fulton DR, et al. Superantigens in Kawasaki syndrome. Clin Immunol Immunopathol. 1995;77:119-126.
12. Arav-Boger R, Assia A, Jurgenson U, Spirer Z. The immunology of Kawasaki disease. Adv Pediatr. 1994;41:359-367.
13. Matsubara T, Farukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990;56:29-36.
14. Barron KS, Shulman ST, Rowley A, et al. Report of the National Institutes of Health workshop on Kawasaki disease. J Rheumatol. 1999;26:170-190.
15. Quasney MW, Bronstein DE, Cantor RM, et al. Increased frequency of alleles associated with elevated tumor necrosis factor-alpha levels in children with Kawasaki disease. Pediatr Res. 2001;49:686-690.
16. Council on Cardiovascular Disease in the Young; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
17. Freeman AF, Shulman ST. Kawasaki disease: summary of the American Heart Association Guidelines. Am Fam Physician. 2006;74:1141-1148.
18. Patel RM, Shulman ST. Kawasaki disease: a comprehensive review of treatment options. J Clin Pharm Ther. 2015;40:620-625.
19. Uehara R, Igarashi H, Yashiro M, et al. Kawasaki disease patients with redness or crust formation at the Bacille Calmette-Guérin inoculation site. Pediatr Infect Dis J. 2010;29:430-433.
20. Nasr I, Tometzki AJ, Schofield OM. Kawasaki disease: an update. Clin Exp Dermatol. 2001;26:6-12.
21. Rosenfeld EA, Corydon KE, Shulman ST. Kawasaki disease in infants less than one year of age. J Pediatr. 1995;126:524-529.
22. Capannari TE, Daniels SR, Meyer RA, et al. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7:355-360.
23. Tsuda E, Kamiya T, Kimura K, et al. Coronary artery dilatation exceeding 4.0 mm during acute Kawasaki disease predicts a high probability of subsequent late intima-medial thickening. Pediatr Cardiol. 2002;23:9-14.
24. Sugimura T, Yokoi H, Sato N, et al. Interventional treatment for children with severe coronary artery stenosis with calcification after long-term Kawasaki disease. Circulation. 1997;96:3928-3933.
25. Kitamura S. The role of coronary bypass operation on children with Kawasaki disease. Coron Artery Dis. 2002;13:437-447.
26. Kato H, Ichinose E, Kawasaki T. Myocardial infarction in Kawasaki disease: clinical analyses in 195 cases. J Pediatr. 1986;108:923-927.
27. Burns JC, Shike H, Gordon JB, et al. Sequelae of Kawasaki disease in adolescents and young adults. J Am Coll Cardiol. 1996;28:253-257.
28. Newburger JW. Kawasaki disease. Curr Treat Options Cardiovasc Med. 2000;2:227-236.
29. Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96:1057-1061.
30. Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997;131:888-893.
31. Furusho K, Kamiya T, Nakano H, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;2:1055-1058.
32. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341-347.
33. Rowley AH, Shulman ST. Pathogenesis and management of Kawasaki disease. Expert Rev Anti Infect Ther. 2010;8:197-203.
34. Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633-1639.
35. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747-2771.
36. Takahashi M, Mason W, Thomas D, Sinatra F. Reye syndrome following Kawasaki syndrome confirmed by liver histopathology. In: Kato H, ed. Kawasaki Disease; Proceedings of the 5th International Kawasaki Disease Symposium; May 22-25, 1995; Fukuoka, Japan. New York, NY: Elsevier Science; 1995:436-444.
37. Lee JH, Hung HY, Huang FY. Kawasaki disease with Reye syndrome: report of one case. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1992;33:67-71.
38. Scheinfeld NS. Kawasaki disease treatment & management. http://emedicine.medscape.com/article/965367-treatment. Accessed January 16, 2016.
39. Burns JC, Capparelli EV, Brown JA, et al. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. 1998;17:1144-1148.
40. Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105:E78.
41. Sundel RP, Burns JC, Baker A, et al. Gamma globulin re-treatment in Kawasaki disease. J Pediatr. 1993;123:657-659.
42. Han RK, Silverman ED, Newman A, McCrindle BW. Management and outcome of persistent or recurrent fever after initial gamma globulin therapy in acute Kawasaki disease. Arch Pediatr Adolesc Med. 2000;154:694-699.
43. Wright DA, Newburger JW, Baker A, Sundel RP. Treatment of immune globulin-resistant Kawasaki disease with pulsed doses of corticosteroids. J Pediatr. 1996;128:146-149.
44. Burns JC, Best BM, Mejias A, et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2008;153:833-838.
45. Oki I, Tanihara S, Ojima T, et al. A multicenter collaborative study on the risk factors of cardiac sequelae due to Kawasaki disease: a one-year follow-up study. Acta Pediatr. 2000;89:1435-1438.
To comment on this article, contact rdavidson@uspharmacist.com.





