US Pharm. 2014;39(12):50-54.
ABSTRACT: Clostridium difficile is emerging as a major public health concern due to an increase in morbidity and mortality associated with C difficile infection (CDI). Many patients with C difficile–associated diarrhea require hospitalization for IV fluid administration and antibiotic therapy directed against C difficile. Metronidazole, vancomycin, and fidaxomicin are the most commonly used antibiotics for the treatment of CDI. Pharmacists from various practice settings play an important role in the prevention and treatment of CDI by educating patients and healthcare professionals about risk factors and the most appropriate therapies based on disease severity and the patient’s characteristics.
Clostridium difficile infection (CDI) leads to 250,000 hospitalizations and 14,000 deaths each year in the United States, according to the CDC.1 There has been a rapid emergence of hypervirulent strains of C difficile, known as NAP1/BI/027 strains, which are often the source of epidemics.2,3 These strains increase toxin production and spore formation, lead to fluoroquinolone resistance, and cause severe disease.2,3 CDI is associated with at least $1 billion in extra healthcare costs each year.1
There are three major guidelines for the diagnosis and treatment of CDI. The Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) joint guidelines were updated in 2010, the American College of Gastroenterology (ACG) in 2013, and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in 2014.4-6 This review is intended to educate pharmacists about the proper management of CDI according to published guidelines and selected studies.
Etiology and Pathophysiology
C difficile is a gram-positive, spore-forming, toxin-producing, anaerobic bacterium that is spread via the fecal-oral route through the ingestion of spores.5 The intestines contain protective flora composed of various types of bacteria that compete for the nutrients and sites within the intestines to survive. An imbalance in the normal flora of the intestines after administration of antibiotics provides an opportunity for C difficile growth and colonization. The invading pathogens attach to the intestinal tract, destroy the mucosa, and produce a variety of toxins. Both toxin A and toxin B are responsible for the release and activation of cytokines in the body, leading to inflammation and diarrhea. However, not all strains of C difficile produce toxins.7
Risk Factors
Risk factors for CDI are summarized in TABLE 1. The single most important risk factor for acquiring CDI is antibiotic administration.4-6 While all antibiotics can potentially cause CDI, clindamycin, cephalosporins, aminopenicillins, and fluoroquinolones are considered high-risk. The risk also increases when the patient receives multiple antibiotics and with prolonged duration of therapy.4-7
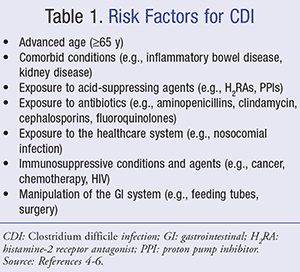
Advanced age is another important risk factor for acquiring CDI. Individuals aged 65 years are at a higher risk of developing CDI possibly because of an increased exposure to the healthcare system and a decline in immune function.6 Other immunocompromised patients such as those with active cancer, HIV patients, and solid-organ transplant recipients are also at increased risk of acquiring CDI.4-6 One controversial risk factor for CDI is the use of acid-suppressing agents.4,5 The use of proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs) has been associated with an increased risk for CDI; however, it is uncertain that there is a direct correlation.8 The transmission of CDI has been shown to be high among healthcare institutions, resulting from environmental contamination or infected patients or employees.5,9
Clinical Presentation and Diagnostic Considerations
Although patients with CDI may be asymptomatic, most individuals present with foul-smelling diarrhea and pseudomembranous colitis, which can sometimes lead to complications such as toxic megacolon, ileus, hypotension, sepsis, or even death.7,10
Diagnosis is based on clinical presentation and laboratory parameters. According to the SHEA/IDSA guidelines, CDI is defined by: 1) presence of diarrhea, with three or more unformed stools within 24 or fewer consecutive hours; and 2) a positive stool test for the presence of toxigenic C difficile or its toxins or pseudomembranous colitis demonstrated through colonoscopic or histopathologic findings.4
Testing for C difficile or toxins should only be performed on the stools of patients with diarrhea. Stool culture is the most sensitive test, yet its slow turnaround time limits its use in practice.4 Sensitivity and specificity of stool culture followed by identification of toxins such as through toxigenic culture by an experienced laboratory is the standard comparator. Although it lacks sensitivity, toxin testing is widely used.4 Another option is to use glutamate dehydrogenase screening tests for C difficile in either a two- or three-step algorithm with subsequent toxin A+B enzyme immunoassay (EIA) testing, or to use cell cytotoxicity assay as confirmatory. However, the sensitivity of such strategies is lower than the nucleic acid amplification tests (NAATs) such as polymerase chain reaction (PCR) for C difficile toxin genes. Repeat testing is discouraged as there are limited data on its utility.4,5
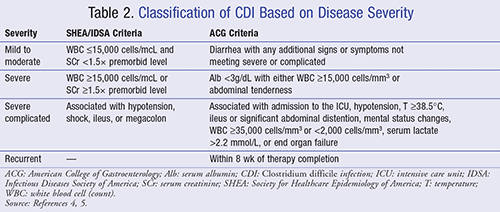
CDI can be classified into four categories based on disease severity. TABLE 2 summarizes the criteria for each severity based on published U.S. guidelines.4,5
Prevention
Early detection leads to early treatment and infection control. Pharmacists from various practice settings can emphasize preventive strategies for CDI. They can educate their patients along with their caregivers and other healthcare workers on complying with proper hand hygiene. C difficile in its spore form is resistant to alcohol; thus, alcohol-based antiseptics will not eradicate the spores. When caring for patients with CDI, hands must be washed with soap and running water.4,5 Contact precaution is another measure pharmacists can counsel upon. Healthcare workers and visitors must use gloves and gowns when caring for or entering the room of a patient with CDI. Contact precaution and private rooms when possible should be used for patients with CDI until the resolution of diarrhea.4,5
Antimicrobial stewardship programs can be designed to reduce the risk of CDI.4,11 Pharmacists can help prevent transmission of C difficile by being cognizant of high-risk patients such as those with recent or current antibiotic therapy or hospitalizations. They can also help minimize exposure and ensure that the appropriate healthcare personnel are informed once a positive C difficile test has resulted, so that optimal therapy and necessary precautions can be initiated.
There are limited data available to recommend the widespread use of probiotics in preventing CDI. Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophiles were found to decrease the risk of CDI in patients >50 years of age who were prescribed antibiotics and were capable of oral intake.4,5,12 However, the study had its limitations, since the sample size was small and high-risk antibiotics were not used. Another small study also found benefit with Lactobacillus acidophilus, yet there are insufficient data to recommend the routine use of probiotics in the prevention of CDI.4,5,13 Other probiotics, such as Lactobacillus rhamnosus GG and Saccharomyces boulardii, have shown a decrease in antibiotic-associated diarrhea but have limited evidence with decreasing CDI.4,5 Although there is moderate evidence for the use of certain probiotics in preventing antibiotic-associated diarrhea, further studies are needed to justify their use in the prevention of CDI.
Treatment
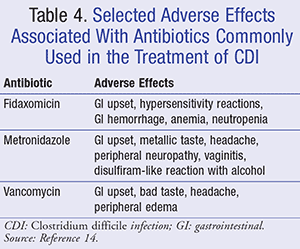
To determine the most appropriate treatment for CDI, several factors must be taken into consideration, including the severity of the episode and patient characteristics. It is generally recommended to discontinue the offending antibiotic(s) when possible, hydrate the patient, avoid antimotility agents, and administer antibiotic therapy specifically targeting C difficile for 10 days. Although it is common practice to prescribe antibiotics for 10 to 14 days, the duration of therapy is 10 days in randomized controlled trials.5 TABLE 3 summarizes antibiotic regimens commonly used in the treatment of CDI, and TABLE 4 details their side-effect profiles.4-6,14

The SHEA/IDSA guidelines for the management of CDI recommend oral metronidazole for an initial mild or moderate episode, oral vancomycin for an initial severe episode, and a combination of high-dose oral (or via nasogastric tube) vancomycin and IV metronidazole, with consideration for rectal instillation of vancomycin if complete ileus, for an initial severe complicated episode.4 These guidelines also recommend the same antibiotic given initially for a first recurrence and vancomycin in a tapered and/or pulsed regimen for a second recurrence.4
The ACG guidelines for the management of CDI recommend oral metronidazole for mild-to-moderate disease with a switch to oral vancomycin if there is no improvement in 5 to 7 days; oral vancomycin for a severe disease; and a combination of high-dose oral vancomycin, IV metronidazole, and high-dose vancomycin enema for a severe complicated disease.5 The same guidelines also recommend to repeat metronidazole or a pulsed vancomycin regimen for recurrent CDI, and to consider fecal microbiota transplantation after three recurrences.5 Fecal transplantation consists of putting healthy donor stool into the stomach, small intestine, or colon of patients with CDI in an attempt to restore normal flora. This approach has been proven to be significantly more effective than vancomycin for the treatment of patients with recurrent CDI, but natural antipathy toward fecal therapy hinders its wide implementation.15
The ESCMID has also published guidelines for the management of CDI.6 It recommends oral metronidazole, vancomycin, or fidaxomicin for an initial nonsevere episode, and oral vancomycin, fidaxomicin, or metronidazole for a severe or complicated episode.6 The same guidelines recommend oral vancomycin, fidaxomicin, or metronidazole for a first recurrence (or if at risk of recurrence), and pulse/taper vancomycin, oral fidaxomicin, high-dose vancomycin, metronidazole, or fecal microbiota transplantation for multiple recurrences.6 When oral administration is not possible, the ESCMID guidelines recommend IV metronidazole for a nonsevere episode, and IV metronidazole plus enteral high-dose vancomycin or IV tigecycline for a severe episode.6
The ESCMID guidelines also recommend probiotics, immunotherapy, and toxin-binding agents in certain cases.6 Toxin-binding is a treatment strategy for CDI that is based on toxin neutralization without antibiotic administration. Although this treatment strategy may seem an attractive option, results from two multinational, randomized, controlled trials have shown that tolevamer, an investigational toxin-binding agent, is inferior to antibiotic treatment for CDI and metronidazole is inferior to vancomycin.16
While metronidazole is widely used in the treatment of C difficile–associated diarrhea (CDAD), vancomycin was until recently the only antibiotic approved by the FDA for this indication.17 Fidaxomicin, a novel macrolide, is the second antibiotic to be approved by the FDA for the treatment of CDAD based on the results of two large randomized, multicenter, double-blind clinical trials demonstrating its noninferiority compared to vancomycin for the treatment of CDI and its superiority in preventing recurrences.18,19 Fidaxomicin has a very narrow spectrum of activity targeted against C difficile and is minimally absorbed, which represent a very attractive option for the treatment of CDI.17 However, because of the high cost of fidaxomicin compared to metronidazole and vancomycin, fidaxomicin should be reserved for the treatment of patients at high risk of recurrence such as the elderly, patients receiving concomitant antibiotic therapy, those with a prior episode of CDI, and patients with immunosuppressive conditions. Other available antibiotics with activity against C difficile but not recommended by the guidelines include nitazoxanide, rifampin, and rifaximin.4-6
Role of the Pharmacist
Pharmacists play an important role in the prevention of CDI by educating patients and healthcare professionals about risk factors for CDI and by emphasizing appropriate infection control strategies and antimicrobial stewardship practices. Pharmacists also play an important role in the treatment of CDI by assisting clinicians in selecting the most appropriate antibiotic regimen based on disease severity and the patient’s characteristics and by counseling patients about possible adverse effects and drug interactions associated with antibiotics directed against C difficile.
REFERENCES
1. CDC. Antibiotic resistance threats in the United States, 2013. www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf#page=6. Accessed August 27, 2014.
2. Hunt JJ, Ballard JD. Variations in virulence and molecular biology among emerging strains of Clostridium difficile. Microbiol Mol Biol Rev. 2013;77(4):567-581.
3. Merrigan M, Venugopal A, Mallozzi M, et al. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. J Bacteriol. 2010;192(19):4904-4911.
4. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
5. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
6. Debast SB, Bauer MP, Kuijper EJ; ESCMID. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(suppl 2):1-26.
7. Gerding DN, Johnson S. Chapter 129. Clostridium difficile infection, including pseudomembranous colitis. In: Longo DL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012. http://accesspharmacy.mhmedical.com/content.aspx?bookid=331&Sectionid=40726873. Accessed August 29, 2014.
8. Barletta JF, El-Ibiary SY, Davis LE, et al. Proton pump inhibitors and the risk for hospital-acquired Clostridium difficile infection. Mayo Clin Proc. 2013;88:1085-1090.
9. Rebmann T, Carrico RM; Association for Professionals in Infection Control and Epidemiology. Preventing Clostridium difficile infections: an executive summary of the Association for Professionals in Infection Control and Epidemiology’s elimination guide. Am J Infect Control. 2011;39:239-242.
10. Bibbò S, Lopetuso LR, Ianiro G, et al. Role of microbiota and innate immunity in recurrent Clostridium difficile infection. J Immunol Res. 2014;2014:462740.
11. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America, the Infectious Diseases Society of America, and the Pediatric Infectious Diseases Society. Infect Control Hosp Epidemiol. 2012;33:322-332.
12. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhea associated with antibiotics: randomised, double-blind, placebo-controlled trial. BMJ. 2007;335(7610):80.
13. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636-1641.
14. Lexi-Comp Online [online database]. Hudson, OH: Lexi-Comp, Inc; 2014. www.lexi.com. Accessed August 1, 2014.
15. Kelly CP. Fecal microbiota transplantation—an old therapy comes of age. N Engl J Med. 2013;368:474-475.
16. Johnson S, Louie TJ, Gerding DN, et al; Polymer Alternative for CDI Treatment (PACT) investigators. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
17. Chahine EB, Sucher AJ, Mantei K. Fidaxomicin: a novel macrolide antibiotic for Clostridium difficile infection. Consul Pharm. 2014;29:614-624.
18. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
19. Cornely OA, Crook DW, Esposito R, et al; OPT-80-004 Clinical Study Group. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12: 281-289.





