ABSTRACT: Guidelines for the management of adults with hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) were recently published by the Infectious Diseases Society of America and the American Thoracic Society. For patients with suspected VAP or HAP, an empiric antimicrobial regimen that has activity against Staphylococcus aureus and Pseudomonas aeruginosa should be used. Empiric coverage for methicillin-resistant S aureus and dual antipseudomonal therapy are needed for patients with risk factors for antimicrobial resistance or for patients being treated in patient-care units demonstrating higher rates of resistance. The recommended duration of antimicrobial treatment for patients with VAP or HAP is 7 days. Pharmacists are in a key position to recommend de-escalation of antimicrobial therapy based on culture and sensitivity results and to ensure that patients are receiving the appropriate duration of therapy.
US Pharm. 2017;42(7)HS-12-HS-26.
Ventilator-associated pneumonia (VAP) is defined as pneumonia that develops in patients receiving mechanical ventilation that occurs at least 48 hours after endotracheal intubation.1 Hospital-acquired pneumonia (HAP) is defined as pneumonia not associated with mechanical ventilation that occurs at least 48 hours after a patient has been admitted to the hospital and that was not incubating at the time of admission.1 A multistate prevalence survey from 2014 found that pneumonia was responsible for 21.8% of healthcare-associated infections.2 Although the mortality rate attributable to VAP varies with patient characteristics, recent data reports an estimated mortality of approximately 10%.3 Additionally, patients with VAP have prolonged durations of mechanical ventilation, intensive-care unit (ICU) stays, and hospital stays, as well as almost $40,000 in excess mean hospitalization costs as compared with patients without VAP.4,5
Guidelines for the management of adults with HAP and VAP were recently published through the collaboration of two societies—the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS).1 Although the concept of healthcare-associated pneumonia was included in the previous version of the guidelines, the 2016 update removed this terminology and recommended its inclusion in future community-acquired pneumonia guidelines, since patients with this type of pneumonia frequently present from the community, are initially cared for in emergency departments, and are not always infected with a multidrug-resistant pathogen. This article focuses on the updated guidelines and reviews the current terminology, etiology, and antimicrobial treatment choices for HAP and VAP.
Diagnosis
The guidelines recommend obtaining cultures of respiratory secretions and blood cultures from all patients with suspected HAP or VAP in order to guide antimicrobial treatment. Noninvasive sampling (such as endotracheal aspiration and sputum expectoration) with semiquantitative culture results (with growth of microorganism(s) reported as light/few, moderate, or abundant/many) is recommended for diagnosis of patients with suspected VAP and HAP. These methods are preferred over invasive bronchoscopic techniques such as bronchoalveolar lavage (BAL) or protected specimen brush (PSB), or noninvasive sampling with quantitative culture results (growth thresholds considered significant at 103 colony-forming units [CFU]/mL for PSB or 104 CFU/mL for BAL), for the diagnosis of HAP or VAP. The rationale for recommending noninvasive sampling with semiquantitative culture results is that these methods can be performed more rapidly, require fewer resources, and are associated with fewer complications than using invasive sampling techniques and reporting quantitative results. For those patients with suspected pneumonia in whom invasive cultures with quantitative results are performed, antibiotics may be discontinued if quantitative culture results are below the diagnostic threshold.1
Additionally, the guidelines recommend using clinical criteria alone (rather than clinical criteria plus procalcitonin [PCT] levels or clinical criteria plus C-reactive protein [CRP] levels) when deciding to initiate antimicrobial treatment for patients with suspected HAP or VAP. The rationale for this recommendation is the inability of CRP to identify patients with VAP and the low sensitivity (67%) and specificity (83%) of clinical criteria plus PCT in diagnosing HAP or VAP.1
TREATMENT
Empiric Therapy
Although the guidelines separate the treatment of VAP and HAP, these infections are treated similarly. Considereations prior to selecting an empiric antimicrobial regimen for a patient with VAP or HAP include patient-specific risk factors for antimicrobial-resistant organisms, history of antibiotic-resistant pathogens, recent antimicrobial exposure, severity of illness, and local susceptibility patterns of pathogens. For patients with suspected VAP or HAP, an empiric antimicrobial regimen should have activity against Staphylococcus aureus and gram-negative organisms, including Pseudomonas aeruginosa. The guidelines recommend that all hospitals regularly create and distribute a local antibiogram that includes antibiotic susceptibility patterns of local pathogens in order to assist clinicians in selecting the optimal empiric antimicrobial regimen for a patient.1
Empiric Treatment for VAP
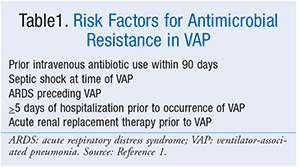
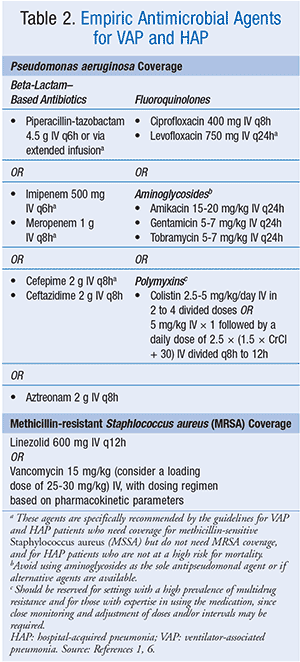
An antimicrobial regimen with activity against S aureus and P aeruginosa should always be administered to patients with clinically suspected VAP. An agent with activity against methicillin-resistant S aureus (MRSA) should be included when the patient has a risk factor for antimicrobial resistance (Table 1) or is being treated in an ICU where greater than 10% to 20% of S aureus isolates are methicillin resistant as determined by the institution-specific antibiogram, or where the prevalence of MRSA is unknown. When empiric MRSA coverage is warranted, either vancomycin or linezolid should be used. For patients with suspected VAP who do not meet criteria for MRSA coverage, the guidelines suggest an empiric regimen that includes piperacillin/tazobactam, cefepime, levofloxacin, imipenem, or meropenem. Although antistaphylococcal penicillins (oxacillin, nafcillin) or cefazolin are preferred for the treatment of proven methicillin-sensitive S aureus (MSSA) pneumonia, they are not necessary to include as part of the initial VAP regimen if one of the recommended empiric agents is used.1Double antipseudomonal antibiotic coverage from two different classes should be considered for the following patients: those with risk factors for antimicrobial resistance (Table 1); those being treated in patient-care units where greater than 10% of gram-negative isolates are resistant to an agent being considered for monotherapy as determined by the institution-specific antibiogram; those being treated in units where local antimicrobial susceptibility rates are unknown; or those with structural lung disease (i.e., bronchiectasis or cystic fibrosis). For patients with suspected VAP who do not have risk factors for antimicrobial resistance, a single antipseudomonal antibiotic may be used if <10% of gram-negative isolates are resistant to that agent. If double gram-negative coverage is required, a beta-lactam–based agent is typically combined with a fluoroquinolone. Although aminoglycosides and polymyxins have activity against gram-negative organisms, aminoglycosides are nephrotoxic and ototoxic and may be associated with lower clinical response rates. Polymyxins are also nephrotoxic and should be reserved for patient-care units with a high prevalence of multidrug resistance. Therefore, the guidelines suggest avoiding these classes if alternate agents are available. Dosing of recommended antimicrobial agents for the treatment of VAP is included in Table 2.1,6
Empiric Treatment for HAP
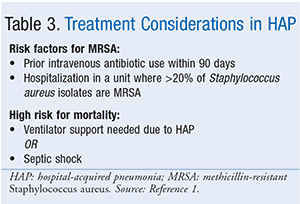
Similar to the treatment of patients with VAP, an antimicrobial regimen with activity against S aureus and P aeruginosa should always be administered to patients with HAP (Table 2). Either vancomycin or linezolid should be used when a patient with suspected HAP has a risk factor for MRSA infection or is at high risk for mortality (Table 3). For empiric treatment of HAP in patients with no risk factors for MRSA infection who are not at high risk for mortality, the guidelines recommend using an antibiotic with activity against MSSA and a regimen that includes piperacillin/tazobactam, cefepime, levofloxacin, imipenem, or meropenem. For patients with HAP who have prior intravenous antibiotic use within 90 days, a high risk for mortality, or structural lung disease (i.e., bronchiectasis or cystic fibrosis), double antipseudomonal coverage with antibiotics from two different classes should be considered. Again, a beta-lactam–based agent is typically used in combination with a fluoroquinolone when double gram-negative coverage is required. Patients who do not meet the criteria for double antipseudomonal coverage may be treated with a single antimicrobial agent that has activity against P aeruginosa. However, the guidelines recommend against using an aminoglycoside as the sole antipseudomonal agent due to poor lung penetration and adverse effect profile.1
De-escalation and Directed Therapy
Once microbiology culture and antimicrobial susceptibility results are reported for patients with VAP or HAP, antimicrobial therapy should be de-escalated and therapy should be directed toward the pathogen(s) causing disease. De-escalation is an important component of antimicrobial stewardship and refers to changing from a broad-spectrum antimicrobial regimen to one that has a narrower spectrum of activity or changing from combination therapy to monotherapy. Directed therapy helps to prevent complications of antimicrobial therapy, including Clostridium difficile infection and the development of antibiotic-resistant bacteria.1
TREATMENT OF SELECTED PATHOGENS
Methicillin-Resistant Staphylococcus aureus
The guidelines recommend using either vancomycin or linezolid for MRSA coverage in patients with HAP or VAP, as they are roughly equivalent for the treatment of MRSA pneumonia.1 Additionally, no alternative agent or regimen has been shown to be clearly superior to either of these agents. The decision to use vancomycin or linezolid should be based upon patient-specific factors, including blood-cell counts (due to linezolid’s risk of anemia, thrombocytopenia, and leukopenia); concurrent use of serotonin-reuptake inhibitors (due to linezolid’s mild, reversible nonselective inhibition of monoamine oxidase, which may lead to serotonin syndrome); renal function (due to a possible increased risk of nephrotoxicity associated with vancomycin when compared with linezolid); and cost (with linezolid being more costly).1,6 If vancomycin is used for the treatment of HAP or VAP, it should be dosed via pharmacokinetics in order to attain a target trough of 15 to 20 mg/L.1
Pseudomonas aeruginosa
Directed therapy for patients with VAP or HAP due to P aeruginosa should be based upon the results of antimicrobial susceptibility testing, since there is a high prevalence of antibiotic resistance in settings where these infections occur. In settings that have a high prevalence of extensively resistant organisms, routine testing for susceptibility to polymyxins should be performed. The guidelines do not have a preferred antipseudomonal antibiotic as there has not been definitive evidence to show that any one antipseudomonal agent is clearly preferable to the others. However, aminoglycosides are not recommended as monotherapy due to their poor penetration into lung alveoli, necessitating high peak serum concentrations (which increase the risk of nephrotoxicity and ototoxicity) and the lack of studies evaluating their use as monotherapy in patients with HAP or VAP. If choosing an antimicrobial agent from the carbapenem class, it is important to note that ertapenem has limited-to-no activity against P aeruginosa and should not be used for treatment of pneumonia caused by this pathogen. In terms of combination therapy for directed treatment of VAP or HAP caused by P aeruginosa, the guidelines suggest using two antimicrobial agents to which the isolate is susceptible for patients who are in septic shock or are at a high risk for death. For patients who do not meet these criteria, a single antibiotic agent to which the isolate is susceptible may be used for treatment.1
Extended-Spectrum Beta-Lactamase–Producing Organisms
Antimicrobial susceptibility testing provides the best information to inform antibiotic choices, as there is no preferred antibiotic regimen for patients with confirmed VAP or HAP caused by gram-negative bacilli that produce extended-spectrum beta-lactamase (ESBL). The guidelines acknowledge that carbapenems are generally considered to be the agents of choice for ESBL infections, but report that there is also data suggesting that piperacillin/tazobactam or cefepime may be appropriate for the treatment of certain isolates. The guidelines state that there is an urgent research need for studies that compare antibiotic regimens for the treatment of pneumonia caused by ESBL-producing organisms.1
Acinetobacter Species
In patients with VAP or HAP caused by Acinetobacter species, the guidelines consider carbapenems, ampicillin/sulbactam, and colistin to be equally effective for treating susceptible isolates. Treatment with either ampicillin/sulbactam or a carbapenem is recommended if the isolate is shown to be susceptible due to the more favorable toxicity profile of these agents as compared with polymyxins. Polymyxins should be reserved for isolates that are susceptible only to this class due to their increased risk for nephrotoxity. If the isolate of Acinetobacter is only sensitive to polymyxins, this class of agents is recommended in conjunction with adjunctive use of aerosolized colistin. The guidelines recommend against the use of tigecycline for the treatment of VAP or HAP caused by Acinetobacter species because this agent is associated with worse clinical outcomes as compared with other therapies.1
DURATION OF THERAPY
The decision to discontinue antibiotic therapy should be based upon clinical criteria (i.e., fever, cough, abnormal findings on lung examination, infiltrates on chest x-ray, tachypnea) in conjunction with a serum PCT level. Although HAP and VAP have traditionally been treated with longer durations of antimicrobial therapy, the current guidelines recommend a 7-day course of antibiotic therapy for the treatment of these types of pneumonias regardless of the pathogen(s) causing infection. Authors of the updated guidelines did not feel that longer durations of therapy were needed for patients with VAP infected with a nonglucose-fermenting gram-negative rod (such as P aeruginosa or Acinetobacter spp.), based on limitations of previous studies that recommended longer durations of treatment and data demonstrating that there were no overall differences in either mortality or clinical cure with shorter courses of therapy. Additionally, shorter treatment courses decrease antibiotic exposure, antibiotic-related side effects, and the potential for antibiotic resistance. It is important to note that the guidelines state that shorter or longer durations of therapy may be needed in certain situations depending upon the rate of improvement of the patient’s clinical, radiologic, and laboratory parameters.1
CONCLUSION
Pharmacists can play an integral role in the management of patients with VAP and HAP. Pharmacists can work with their microbiology laboratory to create an institution-specific antibiogram, regularly update its contents, and educate clinicians about its use. Pharmacists should work with clinicians to select the most appropriate empiric antimicrobial regimen for a patient with VAP or HAP based on the patient’s risk factors for antimicrobial-resistant pathogens. Pharmacists are also in a key position to recommend de-escalation of antimicrobial regimens and to ensure that patients are receiving the appropriate duration of therapy for these types of infections.
REFERENCES
1. Kalili A, Metersky M, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
2. Magill S, Edwards J, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198-1208.
3. Klompas M, Bransoon R, Eichenwald E, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(8):915-936.
4. Muscedere J, Day A, Heyland D. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis. 2010;51(S1):S120-S125.
5. Kollef M, Hamilton C, Ernst F. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol. 2012;33(3):250-256.
6. Lexi-Comp Online [Internet database]. Hudson, OH: Lexi-Comp, Inc; 2017. www.lexi.com. Accessed March 24, 2017.
To comment on this article, contact rdavidson@uspharmacist.com.