US Pharm. 2022;47(4):39-43.
ABSTRACT: According to the CDC, there were an estimated 26 million new sexually transmitted infections (STIs) in the United States in 2018. Approximately one-half of those infections occurred in adolescents and young adults aged 15 to 24 years. Updated guidelines for the treatment of STIs were published by the CDC in 2021. These guidelines contain the most recent evidence-based information related to prevention of STIs, diagnostic testing, and screening, and they provide updated treatment recommendations for gonorrhea, chlamydia, and trichomoniasis. Pharmacists are in a key position to educate patients and healthcare providers about preventive measures and to collaborate with providers in order to ensure appropriate treatment, counseling, and follow-up for the management of STIs.
According to the CDC, the United States had an estimated 26 million new sexually transmitted infections (STIs) in 2018, accounting for almost $16 billion in direct medical costs.1 Approximately one-half of those infections occurred in adolescents and young adults aged 15 to 24 years.1 This article will review the CDC’s 2021 updated treatment guidelines for STIs, with a focus on selected infections and recommendations for their screening and management in adolescents and adults.
Prevention and Control of STIs
The updated CDC guidelines, which contain the most recent evidence-based information related to STIs, discuss strategies for the prevention and control of STIs.2 These include education and counseling on how an individual can avoid STIs; the use of preexposure vaccines (including those for hepatitis A virus, hepatitis B virus, and human papillomavirus [HPV]); identification of both asymptomatic and symptomatic patients with an STI; and appropriate diagnosis, treatment, counseling, and follow-up of STI-infected persons and their sexual partners.2 Additionally, HIV testing is recommended for all patients being evaluated for an STI (unless patient is already known to have HIV), as is assessment for the presence of other STIs and the patient’s eligibility for HIV preexposure prophylaxis.2
Human Papillomavirus
HPV is the most common STI, with an estimated 13 million new infections (diagnosed and undiagnosed) in the U.S. in 2018 and an overall prevalence of approximately 43 million infections.1,3 It is transmitted through oral, vaginal, or anal sexual contact with someone who is infected with the virus.2,3 Most HPV infections are self-limited and asymptomatic.2,3 However, oncogenic types of HPV (such as types 16 and 18) cause the majority of HPV-related cancers, including cervical, penile, anal, vulvar/vaginal, and oropharyngeal cancer, whereas types 6 and 11 are responsible for anogenital warts.2 HPV testing is not recommended for diagnosing warts, but it is used to detect oncogenic strains of HPV as part of cervical cancer screening.2-4
The CDC provides a number of resources supporting vaccination efforts for the prevention of HPV infection and its associated complications.5 The 9-valent HPV vaccine, which protects against types 6, 11, 16, 18, 31, 33, 45, 52, and 58, is recommended as a part of routine immunizations for children aged 11 to 12 years (before exposure to HPV) and for all persons up to age 26 years who have not previously been vaccinated.6-8 In persons with a history of sexual abuse or assault, the vaccine may be administered starting at age 9 years.2,7 For patients aged 27 to 45 years who have not previously been vaccinated, use of the HPV vaccine should be based on shared clinical decision-making between the patient and the provider.6,8 A two-dose series (at 0 and 6-12 months of age) is recommended for immunocompetent persons who start the vaccine series before age 15 years, and three doses (at 0, 1-2 months, and 6 months) are recommended for those who start the series at age 15 years or older and for patients of any age who have an immunocompromising condition.2,6-8
Gonorrhea
The CDC estimates that the U.S. had approximately 1.6 million new cases of gonorrhea in 2018.1 Gonorrhea is a bacterial infection caused by the gram-negative diplococcus bacterium Neisseria gonorrhoeae.1,2,9 N gonorrhoeae can infect the mucous membranes of the reproductive tract, rectum, eyes, and mouth or pharynx, and it can be transmitted perinatally during childbirth.9 Symptoms, if present, include dysuria and urethral or vaginal discharge (for urogenital infections); discharge, anal soreness, bleeding, or painful bowel movements (rectal infections); or a sore throat (pharyngeal infections).9 However, most patients with gonorrhea are asymptomatic or do not develop symptoms until complications (pelvic inflammatory disease [PID] in women, epididymitis in men) have occurred.9
The CDC recommends annual screening for sexually active women younger than age 25 years and for older women at increased risk for infection (new or multiple sexual partners, or a sexual partner with an STI).2,10 For pregnant women, retesting should be performed during the third trimester in those who meet criteria for annual screening.2,10 The CDC also recommends that screening be performed at least annually in men who have sex with men (MSM) or in sexually active persons who are infected with HIV.2,10
Recommended and alternative treatments for uncomplicated urogenital or rectal gonococcal infections are included in TABLE 1.2 Ceftriaxone is the preferred agent for the treatment of pharyngeal infections, and an infectious-disease specialist should be consulted for the management of a patient who has a contraindication to its use.2
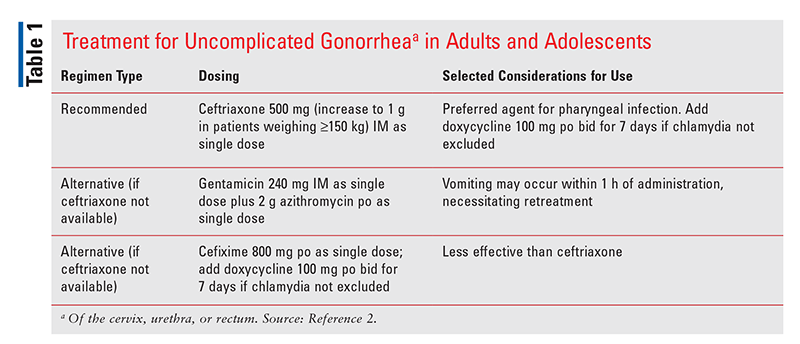
Sexual partners of persons infected with gonorrhea should be referred for evaluation, testing, and treatment if they had sexual contact within 60 days of the patient’s diagnosis or onset of symptoms.2 If it has been more than 60 days since an infected patient’s last sexual contact, the most recent sexual partner should be evaluated and treated.2 It is important to counsel patients to abstain from sexual activity for 7 days after they (and any sexual partners) have received treatment and until resolution of symptoms in order to minimize disease transmission.2
In all patients, retesting should be conducted 3 months after treatment to assess whether a repeat infection is present (reinfection is common); this follow-up visit should be scheduled at the time of initial treatment.2 Additionally, a test of cure should be performed 7 to 14 days after treatment of pharyngeal gonorrhea.2
Chlamydia
Chlamydia, which is caused by the Chlamydia trachomatis bacterium, is the most frequently reported bacterial STI in the U.S., with approximately 4 million new infections occurring in 2018.1,2,11 C trachomatis is either contracted via sexual contact with an infected individual (penile-vaginal, oral, or anal contact) or transmitted perinatally.11 Most patients with chlamydial infection are asymptomatic, but when present, symptoms are similar to those of gonorrhea.11 Untreated chlamydia can cause the following complications: PID (which may be symptomatic or subclinical), ectopic pregnancy, infertility, or reactive arthritis.11
Because most chlamydial infections are asymptomatic, screening tests are important to determine whether a patient is infected.2 The CDC recommends annual screening for sexually active women younger than age 25 years and for older women with an increased risk of infection (new or multiple sexual partners, or a sexual partner with an STI).2,10 For pregnant women, retesting should be performed during the third trimester in those who meet criteria for annual screening.2,10 The CDC also recommends that screening be performed at least annually in MSM and sexually active persons who are infected with HIV.2,10
The recommended regimen for treatment of chlamydia in nonpregnant adolescents and adults is doxycycline 100 mg orally twice daily for 7 days.2 Alternative regimens include azithromycin 1 g orally as a single dose (especially if potential nonadherence to the 7-day doxycycline regimen is a concern) and levofloxacin 500 mg orally daily for 7 days.2 The guidelines state that azithromycin is considered an alternative agent because it may be less effective for treating rectal chlamydia, which frequently co-occurs with urogenital infections; if it is used, providers should consider posttreatment evaluation of the treated patient.2 For pregnant patients, the recommended regimen for chlamydia treatment is azithromycin 1 g orally as a single dose; amoxicillin 500 mg orally three times daily for 7 days is an alternative regimen.2
Sexual partners of persons infected with chlamydia should be referred for evaluation, testing, and treatment if they had sexual contact within 60 days of the patient’s diagnosis or onset of symptoms.2 For patients who have not had sexual contact within the prior 60 days, the most recent sexual partner should be evaluated and treated.2 It is important to counsel patients to abstain from sexual activity for 7 days after they (and any sexual partners) have received treatment with a single dose of azithromycin or until completion of a 7-day treatment regimen and until resolution of any symptoms.2
In all patients, retesting should occur approximately 3 months after treatment in order to assess for the presence of a repeat infection (reinfection is common); this follow-up visit should be scheduled at the time of initial treatment.2 Additionally, in pregnant patients it is recommended that a test of cure be performed about 4 weeks after completion of therapy in order to confirm eradication of the infection.2
Trichomoniasis
Trichomoniasis is caused by Trichomonas vaginalis, a protozoan parasite that resulted in more than 2 million new infections in the U.S. in 2018.1,12 Approximately 70% to 85% of persons infected with trichomoniasis are asymptomatic or have minimal symptoms.12 Symptoms, if present, include genital itching, burning, or soreness; discomfort during urination or intercourse; and penile or vaginal discharge that in females is characterized by a “fishy” odor.12 Trichomoniasis infection can cause genital inflammation, which may increase the patient’s risk for transmitting or becoming infected with another STI; additionally, it may cause preterm delivery or delivery of a low-birthweight baby in pregnant women.12 Because of its high prevalence, it is recommended to test for T vaginalis in all females seeking medical care for vaginal discharge.2 Additionally, routine annual screening for trichomoniasis is recommended for HIV-infected females because of the high prevalence of coinfection and the potential for PID and other adverse health events in this patient population.2,10
Recommended and alternative treatments for trichomoniasis are summarized in TABLE 2.2,13,14 Another nitroimidazole agent, secnidazole, was approved in June 2021 as single-dose oral therapy for the treatment of trichomoniasis in adult patients and sexual partners.15 This agent is supplied as a 2-g packet of granules (taken without regard to timing of meals) that must be sprinkled onto applesauce, yogurt, or pudding and entirely consumed within 30 minutes. Patients should be counseled to avoid alcohol during treatment and for at least 2 days after completion of secnidazole therapy.15
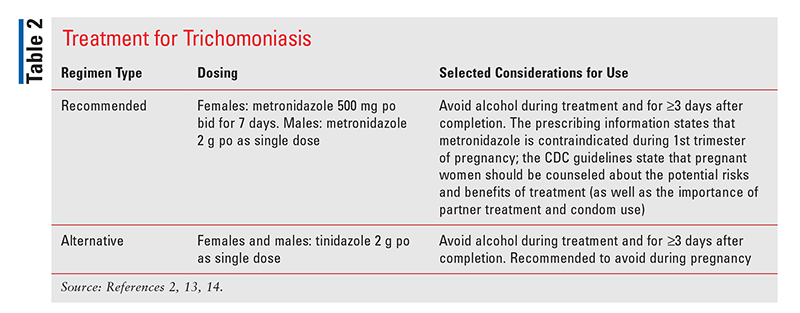
Current sexual partners of persons infected with trichomoniasis should be referred for evaluation and treatment.2 For recurrent infections in individuals who have been reexposed to an untreated partner, a repeat course of the same regimen used for initial treatment is recommended.2 If the recurrent infection is not due to reexposure, it is recommended that females be treated with metronidazole or tinidazole 2 g once daily for 7 days and that males be treated with metronidazole 500 mg twice daily for 7 days.2 It is important to counsel patients to abstain from sexual activity until after they (and any sexual partners) complete therapy and achieve resolution of any symptoms.2 Retesting should occur in all sexually active females within 3 months after initial treatment, since reinfection with trichomoniasis is common in females who were previously treated.2
Genital Herpes
Two types of herpes simplex virus (HSV) have been identified as causing genital herpes: HSV type 1 (HSV-1) and HSV type 2 (HSV-2).16 Although HSV-1 most commonly causes oral herpes characterized by cold sores or fever blisters, it can be transmitted through oral sex, resulting in a genital infection.16,17 HSV-2 most commonly causes genital herpes (with >500,000 cases estimated in the U.S. in 2018) and is typically contracted via genital sexual contact.1,16,17 HSV may also be transmitted during pregnancy or delivery, causing neonatal herpes.2,16
Most primary HSV infections are asymptomatic or mildly symptomatic.16 When symptomatic, a first episode of genital herpes is characterized by blister-like vesicles that ultimately break open, resulting in painful, ulcerative lesions.16,17 First episodes may also be associated with systemic influenza-like symptoms.16,17 Because HSV infections are chronic and lifelong, recurrent outbreaks are common, and recurrent genital infections are more likely to occur in persons infected with HSV-2 than in those with HSV-1.16,17 Recurrent infections usually have a shorter duration and milder symptoms than primary infections, and they may be associated with a prodrome characterized by localized pain or burning/tingling that manifests prior to the appearance of lesions.16,17 It is important to note that HSV may be transmitted even in the absence of visible lesions.16,17
The CDC does not recommend routine screening for HSV-1 or HSV-2 in the general population.2,16 The recommended treatments for a primary or recurrent episode of genital herpes, as well as recommendations for daily suppressive therapy, are included in TABLE 3.2 For patients with severe disease or who have a complication of infection requiring hospitalization, IV acyclovir is recommended.2
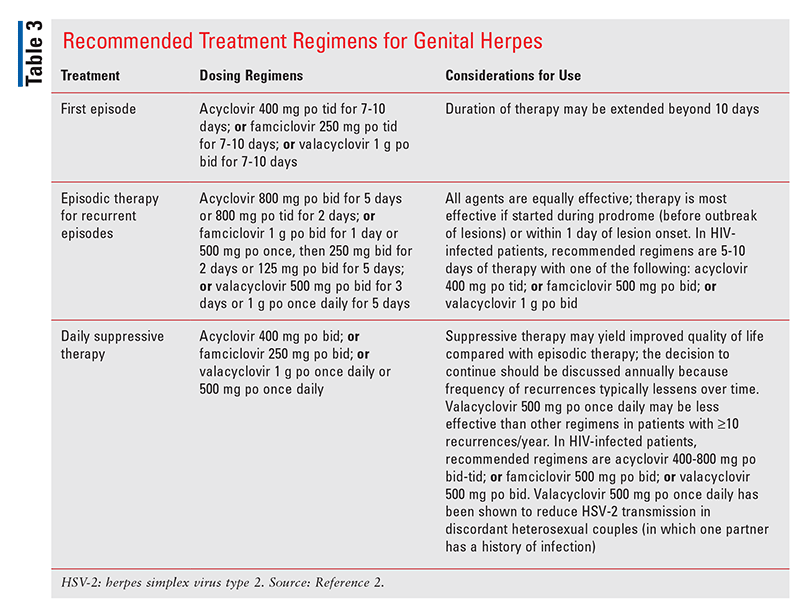
Although systemic antiviral agents are beneficial for controlling the signs and symptoms of genital herpes as well as preventing recurrence and transmission to sexual partners, patients should be counseled that genital herpes is a chronic infection for which there is no cure.2 Suppressive therapy (in addition to consistent use of latex condoms) should be considered in order to prevent transmission to sexual partners.2 Because of the risk of perinatal transmission, women should be counseled to avoid contracting HSV during pregnancy, including abstaining during the third trimester from sexual contact with anyone known or suspected to have herpes.2,16 In patients who have persistent lesions or lesions that recur during appropriate antiviral treatment, it is recommended to obtain a viral culture to assess for acyclovir resistance.2
The Pharmacist’s Role
Pharmacists are ideally placed to educate patients as well as healthcare providers about measures that can be undertaken to prevent STIs, including use of the HPV vaccine. Pharmacists are also well positioned to collaborate with clinicians in order to ensure appropriate management of infected patients and sexual partners.
REFERENCES
1. CDC. Sexually transmitted infections prevalence, incidence, and cost estimates in the United States. www.cdc.gov/std/statistics/prevalence-2020-at-a-glance.htm. Accessed December 8, 2021.
2. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1-187.
3. CDC. Genital HPV infection—fact sheet. www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed December 8, 2021.
4. Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70(5):321-346.
5. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/index.html. Accessed December 8, 2021.
6. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698-702.
7. CDC. Child and adolescent immunization schedule: recommendations for ages 18 years or younger, United States, 2022. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed March 1, 2022.
8. CDC. Adult immunization schedule: recommendations for ages 19 years or older, United States, 2022. www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed March 1, 2022.
9. CDC. Gonorrhea—CDC fact sheet (detailed). www.cdc.gov/std/gonorrhea/stdfact-gonorrhea-detailed.htm. Accessed December 8, 2021.
10. CDC. Screening recommendations and considerations referenced in treatment guidelines and original sources. www.cdc.gov/std/treatment-guidelines/screening-recommendations.htm. Accessed December 8, 2021.
11. CDC. Chlamydia—CDC fact sheet (detailed). www.cdc.gov/std/chlamydia/stdfact-chlamydia-detailed.htm. Accessed December 8, 2021.
12. CDC. Trichomoniasis—CDC fact sheet. www.cdc.gov/std/trichomonas/stdfact-trichomoniasis.htm. Accessed December 8, 2021.
13. Flagyl (metronidazole) package insert. New York, NY: Pfizer Inc; December 2021.
14. Tindamax (tinidazole) package insert. San Antonio, TX: Mission Pharmacal Co; June 2019.
15. Solosec (secnidazole) package insert. Baltimore, MD: Lupin Pharmaceuticals, Inc; June 2021.
16. CDC. Genital herpes—CDC fact sheet (detailed). www.cdc.gov/std/herpes/stdfact-herpes-detailed.htm. Accessed December 8, 2021.
17. CDC. Genital herpes–CDC fact sheet. www.cdc.gov/std/herpes/stdfact-herpes.htm. Accessed December 8, 2021.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





