US Pharm. 2018;43(6):HS2-HS8.
ABSTRACT: The incidence of melanoma continues to increase exponentially. It was estimated that, in 2016, 76,380 people in the United States would be diagnosed with melanoma and about 10,130 would die of this disease. Survival rates have started to improve because of new and emerging systemic therapies, including immunotherapy and targeted therapy. The National Comprehensive Cancer Network has updated its guidelines on the use of immunotherapy and targeted therapy in melanoma. Pharmacists can help the clinician select the most appropriate therapy and can actively conduct patient education.
It was calculated that, in 2016, 76,380 people in the United States would be diagnosed with melanoma and about 10,130 would die of the disease. It is estimated that an individual loses 20.4 years of potential life as a result of melanoma, compared with 16.6 years for all malignancies.1 This exponential increase is more rapid than for other malignancies in both men and women, with the exception of lung cancer in women.2 Survival rates in patients with melanoma, like those for most malignancies, depend on the stage at presentation. In the United States, 84% of patients with melanoma present with localized disease, 9% with regional disease, and 4% with distant metastatic disease.3 Five-year survival rates range from 90% for localized disease with various parameters to less than 10% for distant metastatic melanoma.4,5 However, emerging and effective systemic therapies, along with targeted therapies for specific genetic alterations in distinct clinical subtypes of melanoma, have increased the possibility of long-term remission for a larger number of patients.6
Treatment Overview
Initial treatment for primary melanoma continues to involve surgical excision and may include adjuvant radiation to help reduce the risk of recurrence. For patients with stage III in-transit melanoma in whom resection is not feasible, nonsurgical local approaches may include intralesion injections.5
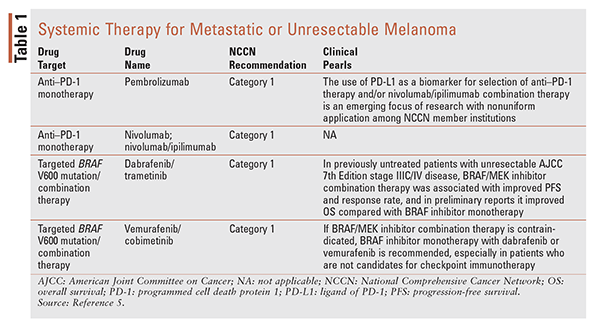
For first-line treatment of unresectable or metastatic disease (TABLE 1), recommended options include checkpoint immunotherapy, BRAF-targeted therapy (in the case of BRAF-mutated disease), and clinical trials.5 For disease-free resectable melanoma or advanced disease, recent studies have supported new adjuvant treatments based on their superior efficacy compared with traditional chemotherapy. Two new treatments include biochemotherapy—a combination of high-dose interferon, interleukin-2 (IL-2), and chemotherapy—and immune checkpoint inhibitors.7,8 For patients with BRAF-mutant metastatic disease, BRAF-targeted treatments may include BRAF/MEK inhibitor combination therapy with dabrafenib/trametinib or vemurafenib/cobimetinib or single-agent BRAF-inhibitor therapy with vemurafenib or dabrafenib.5 Treatment selection in any stage depends on many factors, including the patient’s overall health, comorbidities, and risk of recurrence, and risks and benefits should be assessed before treatment initiation.
Nivolumab
Cancer cells may develop the ability to escape immunosurveillance (the body’s ability to identify and destroy malignant cells). Checkpoint immunotherapies help increase immune response to circumvent these evasion mechanisms.9-11 Monoclonal antibodies against the immune checkpoint receptor programmed cell death protein 1 (PD-1), such as nivolumab (Opdivo), prevent the receptor-ligand interaction on tumor cells, allowing T-cell activation and adequate immune response.12 Nivolumab, a human immunoglobulin G4 (IgG4) and PD-1 inhibitor, is recommended by the National Comprehensive Cancer Network (NCCN) as first-line therapy for unresectable and metastatic melanoma (category 1).5
The efficacy of nivolumab in previously untreated unresectable stage III or IV melanoma was investigated in two phase III clinical trials. In CheckMate-066, nivolumab improved response rate, progression-free survival (PFS), and overall survival (OS) compared with chemotherapy, and percentages of grade 3 and 4 adverse effects (AEs) were lower for nivolumab versus chemotherapy.13 CheckMate-067 was a randomized, double-blind, phase III study that compared nivolumab alone or with ipilimumab versus ipilimumab alone in patients with metastatic melanoma.14 In this trial, nivolumab monotherapy improved response rates and PFS compared with single-agent ipilimumab, and it was associated with lower toxicity.14 Both trials demonstrated that, in the first-line setting, nivolumab is a better option than chemotherapy or ipilimumab for patients with unresectable or metastatic disease.
Nivolumab was recently approved for a supplemental Biologics License Application to update the dosing schedule to include 480 mg infused every 4 weeks for a majority of approved indications, in addition to the already available option of 240 mg every 2 weeks.15 Significant toxicities—which are immune-mediated because of the drug’s mechanism of action—include hypothyroidism or hyperthyroidism, pneumonitis, nephritis, hepatitis, endocrinopathies, and pituitary disorders.16 Although the frequency of grade 3 and 4 AEs requiring management is lower with anti–PD-1 monotherapy than with ipilimumab-containing regimens, AE management involves withholding or discontinuing treatment and administering systemic corticosteroids.5
Ipilimumab
Although both anticytotoxic T lymphocyte–associated protein 4 (CTLA4) (ipilimumab) and anti–PD-1 (nivolumab and pembrolizumab) agents are checkpoint immunotherapies, they are not considered to have the same mechanism of action because they target different molecules. Adjuvant ipilimumab was FDA-approved for a prolonged high-dose regimen: 10 mg/kg every 3 weeks for four doses, followed by 10 mg/kg every 12 weeks for up to 3 years or until documented disease recurrence or unacceptable toxicity. For treatment of unresectable or metastatic disease, the recommended ipilimumab dosage is much lower (3 mg/kg) and the treatment duration much shorter (every 3 weeks for a total of four doses), consistent with the dosing regimen in the phase III trials discussed below.17
Two phase III trials in patients with unresectable stage III or IV melanoma support the use of ipilimumab for advanced disease.18,19 Ipilimumab is approved for treatment of unresectable or metastatic melanoma, including treatment-naïve and previously treated disease.5 However, single-agent ipilimumab monotherapy is no longer an NCCN-recommended first-line option, based on CheckMate-067, which showed improved outcomes with anti–PD-1 monotherapy or nivolumab/ipilimumab combination therapy versus ipilimumab monotherapy.5 FDA-recommended dosing regimens indicate that, for all three approved regimens containing anti–PD-1 agents, treatment should continue until disease progression or unacceptable toxicity.20
Because of a lack of data on long-term anti–PD-1 treatment, the optimal treatment duration is unknown. In the absence of unacceptable toxicity, it is common practice to continue until maximal response is seen. Although there is not a standard definition, maximal response is commonly defined as lack of additional tumor regression on at least two consecutive scans taken at least 12 weeks apart.5
Treatment-related AEs occur in a high percentage of patients treated with anti-CTLA4 or anti–PD-1 agents, and grade 3- or 4-related AEs occur in up to 20% of those receiving single-agent therapy and approximately 50% of those receiving ipilimumab monotherapy or nivolumab/ipilimumab combination therapy.18 As with nivolumab, treatment-related AEs are autoimmune in nature. Close monitoring for potentially lethal immune-related AEs (irAEs) in patients receiving ipilimumab is essential. Ipilimumab is associated with a variety of irAEs, and the frequency and severity of these toxicities increase with increasing doses.21 The most common AEs are cutaneous toxicities (rash, pruritus, and vitiligo), gastrointestinal toxicities (diarrhea/colitis), and fatigue.5 The most common high-grade toxicities observed in clinical trials were endocrinopathies (e.g., hypophysitis, hypo- or hyperthyroidism) and hepatitis (e.g., elevated alanine aminotransferase/aspartate aminotransferase).17 With the exception of endocrinopathies, immune-related AEs resolved when they were managed by withholding ipilimumab and administering corticosteroids.22
Ipilimumab/Nivolumab Combination Therapy
Two randomized trials demonstrated that combination therapy with ipilimumab and nivolumab significantly improved response and PFS compared with ipilimumab monotherapy in patients with previously untreated unresectable stage III or IV disease.14,22 Further follow-up is needed to determine whether nivolumab/ipilimumab combination therapy improves OS compared with single-agent ipilimumab. In patients with BRAF-mutant tumors, however, subgroup analyses in CheckMate-067 and -069 showed improved efficacy with nivolumab/ipilimumab combination therapy versus ipilimumab monotherapy regardless of BRAF-mutation status.14 Both trials also showed substantially increased toxicity with immune checkpoint combination therapy versus monotherapy.
Based on these results, the FDA-recommended dosing regimen for nivolumab/ipilimumab combination therapy is nivolumab 1 mg/kg followed by same-day ipilimumab 3 mg/kg, every 3 weeks for four doses, and then single-agent nivolumab 3 mg/kg every 2 weeks until disease progression or toxicity.17,23 Safety results from randomized phase II and III trials showed that combination therapy with nivolumab and ipilimumab was associated with higher rates of toxicity compared with single-agent ipilimumab or nivolumab.14,20 Ipilimumab/nivolumab combination therapy also demonstrated an increase in total number of patients with treatment-related AEs of any grade and notably increased the occurrence of grade 3 or 4 AEs and AEs leading to treatment discontinuation (36 vs. 8; 15%).20 Of all toxicities commonly observed with immune checkpoint inhibitors, grade 3 and 4 AEs occurred more frequently with combination therapy than with either monotherapy. Many high-grade or refractory irAEs have been successfully managed with high-dose oral or IV corticosteroids, and immunosuppressants have been beneficial in some particularly challenging cases of gastrointestinal and hepatic irAEs.24
Although the nivolumab/ipilimumab combination regimen is an NCCN first-line recommendation (category 1), selection of anti–PD-1 monotherapy or nivolumab/ipilimumab combination therapy should be made based on the consideration that although the combination provides somewhat better PFS, it is associated with a much higher risk of serious immune-mediated toxicities.
Pembrolizumab
Pembrolizumab, another human IgG4 and PD-1 inhibitor, is in the same category as nivolumab and is also recommended by the NCCN as first-line therapy for unresectable and metastatic melanoma (category 1).5 As with nivolumab, pembrolizumab has demonstrated improved response and PFS compared with chemotherapy or ipilimumab (monotherapy), and in clinical trials it has been associated with a lower risk of AEs in patients with unresectable stage III or IV metastatic disease.25 The KEYNOTE-006 trial demonstrated that pembrolizumab results in improved OS compared with ipilimumab.25
The FDA-recommended dosage of pembrolizumab is 2 mg/kg every 3 weeks until disease progression or unacceptable toxicity.26 Although studies such as KEYNOTE-002 administered pembrolizumab for a maximum of 24 months,27 it is common for patients to discontinue anti–PD-1 therapy earlier than that. Clinical experience with treatment beyond 1 year is limited, and further studies should be performed to determine whether lower-frequency maintenance therapy is sufficient to maintain long-term clinical benefit.5 Currently, no data from prospective, randomized trials directly compare nivolumab and pembrolizumab, but these agents appear to have similar safety profiles. Labeling for both nivolumab and pembrolizumab includes specific warnings about pneumonitis and nephritis.23,26 However, randomized clinical trials have shown that single-agent nivolumab and pembrolizumab are associated with less toxicity than ipilimumab monotherapy. Compared with ipilimumab, both anti–PD-1 immunotherapies were associated with notably less diarrhea and pruritus, but more hypothyroidism.14,25 Endocrinopathies associated with ipilimumab are more difficult to manage and require hormone replacement therapy (HRT) in addition to corticosteroids.5 Compared with other irAEs associated with ipilimumab, endocrinopathies were less likely to fully reverse and took longer to resolve.28,29 Patients with endocrinopathies frequently required ongoing HRT, highlighting the importance of early detection to minimize long-term effects.
Talimogene Laherparepvec
For patients in whom complete surgical excision to clear margins is not feasible, clinical trial treatment is the first-line recommendation. Other local, regional, or systemic therapies may be considered.5 Patients with a limited number of in-transit metastases, particularly dermal lesions, which are not receptive to complete surgical excision, should be considered for intralesional local injections.5 Intralesional injection of talimogene laherparepvec (T-VEC) is recommended by the NCCN as first-line therapy in patients with unresectable stage III in-transit disease based on improved durable response rate (DRR) and overall response rate (ORR) versus injection with granulocyte macrophage colony-stimulating factor (GM-CSF) alone.5
Intralesional injection of melanoma metastases with GM-CSF resulted in modest response rates or stable disease in several small clinical studies.30-33 These and other studies led to the development of T-VEC, which uses a modified herpes simplex virus to induce tumor-cell lysis and deliver localized expression of GM-CSF to injected lesions.34 In a recent phase III trial, selected patients with unresectable stage IIIB-IV melanoma were randomized to intralesional injection of T-VEC versus SC injection of GM-CSF.35 Patients had at least one cutaneous, SC, or nodal lesion or aggregation of lesions greater than 10 mm in diameter, measurable disease, and limited distant metastatic disease. T-VEC produced clinically significant DRRs in injected tumors, as well as a bystander effect on some uninjected nonvisceral and visceral tumors.36 At median follow-up of approximately 44 months, T-VEC patients had higher DRRs (16.3% vs. 2.1%, P <.001) and ORRs (26.4% vs. 5.7%, P <.001) than GM-CSF patients.35 In subset analyses, the effect of T-VEC on response rate was greater in patients with less-advanced disease. T-VEC patients with stage IIIB or IIIC disease had a DRR of 33% compared with 0% for GM-CSF patients; for patients with stage IV-M1a disease, the effect of T-VEC on DRR was smaller (16.0% vs. 2.3%). The effect of T-VEC on DRR was far greater in patients with previously untreated metastatic disease (23.9% vs. 0%) than in those with previously treated disease (9.6% vs. 5.6%). Common toxicities were fatigue, chills, pyrexia, nausea, flulike illness, injection-site pain, and vomiting; treatment-related grade 3-4 toxicities, which occurred in 11% of patients, included injection-site reactions (e.g., cellulitis, pain, peripheral edema) and systemic toxicities (fatigue, vomiting, and other flulike symptoms).35
If T-VEC is not available, intralesional injection with IL-2 is another option, as is injection with bacillus Calmette-Guérin or interferon.5 These options are NCCN category 2B recommendations.
Dabrafenib/Trametinib Combination Therapy
Approximately one-half of patients with metastatic cutaneous melanoma harbor an activating mutation of BRAF, an intracellular signaling kinase in the MAP kinase pathway.37-39 Dabrafenib and vemurafenib were developed to inhibit BRAF with mutations at V600.40,41 Despite high initial response rates, one-half of patients with previously untreated stage IV or unresectable stage III melanoma receiving BRAF-targeted monotherapy relapse within about 6 months because of the development of drug resistance.42-44 To bypass possible BRAF-inhibitor therapy resistance, other drugs have been developed to target the MAP kinase pathway in different ways. Trametinib and cobimetinib are oral small-molecule inhibitors of MEK1 and MEK2, signaling molecules downstream of BRAF in the MAP kinase pathway. Although MEK-inhibitor monotherapy has limited utility for treating advanced metastatic melanoma, phase III trials have demonstrated that combination therapy with a BRAF/MEK inhibitor has better efficacy than BRAF-inhibitor monotherapy for previously untreated unresectable or metastatic disease.45,46 Compared with single-agent dabrafenib or vemurafenib, combination therapy with dabrafenib and trametinib improved response rate and duration, PFS, and OS.45,46
These studies have established dabrafenib/trametinib combination regimens as FDA-approved options for the treatment of unresectable or metastatic melanoma with BRAF V600E or V600K mutations.47-49 In phase III trials, common toxicities associated with BRAF-inhibitor monotherapy were fatigue, arthralgia or myalgia, pyrexia and chills, cutaneous events, alopecia, and cutaneous AEs.45,46 Skin complications that occurred with notable prevalence, severity, and variety included not only rash, pruritus, and photosensitivity, but also keratoacanthomas, cutaneous squamous-cell carcinomas, papillomas, hyperkeratoses, and actinic keratosis. For patients receiving BRAF-inhibitor therapy, the panel recommends regular dermatologic evaluation with referral to a dermatologist to monitor for skin complications.8
As is the case with dabrafenib/trametinib, vemurafenib/cobimetinib combination regimens are approved to treat patients who have unresectable or metastatic melanoma with BRAF V600E or V600K mutations. However, further follow-up is needed to determine whether vemurafenib/cobimetinib also improves OS. Among recommended BRAF-targeted therapy options, the BRAF/MEK inhibitor combination is preferred over BRAF inhibitor monotherapy based on improved outcomes and similar risk of toxicity. In patients with documented BRAF V600 mutations, choosing between first-line checkpoint immunotherapy and BRAF-targeted therapy can be difficult given the lack of comparative phase III clinical trials.5 Clinical trials are under way to address questions regarding the optimal sequencing and/or combination of these agents.
Conclusion
Treatment for advanced melanoma has significantly evolved to include newer and more effective systemic therapies, targeted therapies for specific genetic alterations in distinct clinical subtypes of melanoma, and local therapies. Clinical studies have established recommended treatment options including checkpoint immunotherapy, BRAF-targeted therapy for patients with BRAF-mutated disease, and clinical trials as first-line therapy for unresectable or metastatic disease. Current guidelines have been updated to reflect these changes, and they continue to transform as new therapies are developed. Pharmacists can play a role in this ever-changing environment by helping the clinician select therapy based on appropriateness and side-effect profile. Pharmacists can also actively conduct patient-education sessions and advise patients on how to handle medication side effects.
REFERENCES
1. Ekwueme DU, Guy GP Jr, Li C, et al. The health burden and economic costs of cutaneous melanoma mortality by race/ethnicity—United States, 2000 to 2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S133-S143.
2. National Cancer Institute. Cancer stat facts: melanoma of the skin. http://seer.cancer.gov/statfacts/html/melan.html#ref11. Accessed February 19, 2018.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29.
4. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines). Melanoma. Version 2.2018. www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed May 3, 2018.
6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
7. Flaherty LE, Othus M, Atkins MB, et al. Southwest Oncology Group S0008: a phase III trial of high-dose interferon Alfa-2b versus cisplatin, vinblastine, and dacarbazine, plus interleukin-2 and interferon in patients with high-risk melanoma—an intergroup study of cancer and leukemia Group B, Children’s Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. J Clin Oncol. 2014;32:3771-3778.
8. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
9. Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23(suppl 8):viii6-viii9.
10. Bhatia A, Kumar Y. Cellular and molecular mechanisms in cancer immune escape: a comprehensive review. Expert Rev Clin Immunol. 2014;10:41-62.
11. Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(suppl):S185-S198.
12. Peggs KS, Quezada SA, Chambers CA, et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717-1725.
13. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
14. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
15. Opdivo (nivolumab) package insert. Princeton, NJ: Bristol-Myers Squibb Co; April 2018.
16. Opdivo (nivolumab) package insert. Princeton, NJ: Bristol-Myers Squibb Co; February 2017.
17. Yervoy (ipilimumab) package insert. Princeton, NJ: Bristol-Myers Squibb Co; October 2015.
18. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
19. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
20. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
21. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
22. Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675-1682.
23. Opdivo (nivolumab) injection package insert. Princeton, NJ: Bristol-Myers Squibb Co; April 2018.
24. De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res. 2015;25:321-327.
25. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521-2532.
26. Keytruda (pembrolizumab) package insert. Whitehouse Station, NJ: Merck & Co, Inc; September 2017.
27. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
28. Min L, Hodi FS, Giobbie-Hurder A, et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2015;21:749-755.
29. Faje AT, Sullivan R, Lawrence D, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99:4078-4085.
30. Ridolfi L, Ridolfi R. Preliminary experiences of intralesional immunotherapy in cutaneous metastatic melanoma. Hepatogastroenterology. 2002;49:335-339.
31. Si Z, Hersey P, Coates AS. Clinical responses and lymphoid infiltrates in metastatic melanoma following treatment with intralesional GM-CSF. Melanoma Res. 1996;6:247-255.
32. Nasi ML, Lieberman P, Busam KJ, et al. Intradermal injection of granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with metastatic melanoma recruits dendritic cells. Cytokines Cell Mol Ther. 1999;5:139-144.
33. Hoeller C, Jansen B, Heere-Ress E, et al. Perilesional injection of r-GM-CSF in patients with cutaneous melanoma metastases. J Invest Dermatol. 2001;117:371-374.
34. Kaufman HL, Ruby CE, Hughes T, Slingluff CL Jr. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer. 2014;2:11.
35. Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780-2788.
36. Andtbacka RH, Chastain M, Li A, et al. Phase 2, multicenter, randomized, open-label trial assessing efficacy and safety of talimogene laherparepvec (T-VEC) neoadjuvant treatment (tx) plus surgery vs surgery for resectable stage IIIB/C and IVM1a melanoma (MEL). ASCO Meeting Abstracts. 2015;33:TPS9094.
37. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
38. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
39. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279-3290.
40. Lemech C, Infante J, Arkenau HT. The potential for BRAF V600 inhibitors in advanced cutaneous melanoma: rationale and latest evidence. Ther Adv Med Oncol. 2012;4:61-73.
41. Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190-200.
42. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358-365.
43. Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707-714.
44. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
45. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444-451.
46. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
47. GlaxoSmithKline. Prescribing information: Tafinlar (dabrafenib) package insert. 2015. East Hanover, NJ: Novartis Pharmaceuticals Corp; April 2018.
48. Cotellic (cobimetinib) package insert. South San Francisco, CA: Genentech; January 2018.
49. Mekinist (trametinib) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; April 2018.
To comment on this article, contact rdavidson@uspharmacist.com.





