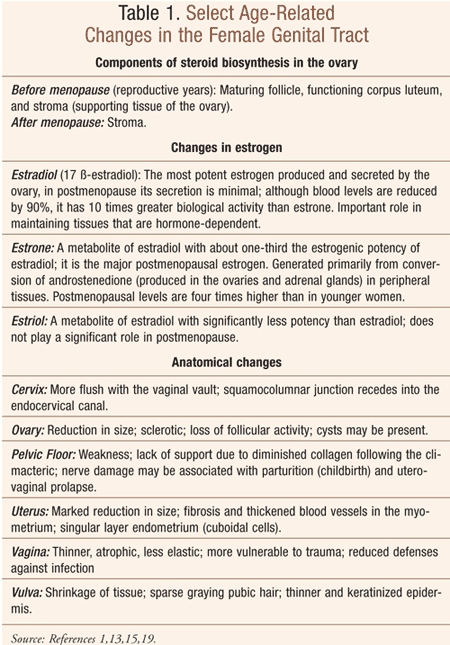
The physiological aging process in females accelerates after menopause, with changes occurring secondary to the cessation of estrogen production in the ovaries.1 These changes occur particularly with regard to the genital tract (Table 1). Atrophic vaginitis (AV, vaginal atrophy), thinning and inflammation of the vaginal walls secondary to a decline in estrogen, is experienced by almost 50% of postmenopausal women.2-4 It is associated with vaginal burning, itching, irritation, and dryness and/or discomfort/pain during intercourse (dyspareunia).3,5 The urinary tract is also affected by postmenopausal declining estrogen, which may lead to thinning of the bladder and urethral linings and possibly cause chronic dysuria and an increased incidence of urinary tract infections.4,6 Overall, the health of the vaginal lining and urethral tissues is greatly influenced by the hormonal environment of a woman’s body.
While AV is a common problem, it is often overlooked.7 Because a discussion about painful intercourse is a sensitive one, many women may be inhibited about broaching the subject with their health care provider or seeking treatment.4,6 Further, many women believe their symptoms are unavoidable signs of aging. Pearson notes the importance of raising the awareness of all health care providers who care for women in their postmenopausal years regarding the considerable incidence of AV and the necessity of being proactive in questioning women about classic symptoms.4,6 In light of the baby boom surge, there is a significant potential to improve the quality of life for this large group of women; additionally, the demand for professional advice and guidance in this regard is sure to increase. Pharmacists should familiarize themselves with the necessary information regarding AV, atrophic urethritis, and the management of urogenital health in postmenopausal women. Providing information to patients about the condition and recommending nonpharmacologic and pharmacologic management interventions are imperative (Table 2, Resources).
Atrophic Vaginitis
Physiological and structural changes that occur within the vulvovaginal mucosa lead to the condition commonly called AV (Table 1).8 Subsequent to a marked postmenopausal decline in estrogen, or a loss of estrogen secondary to some treatments (e.g., oophorectomy, pelvic radiation, certain chemotherapy drugs), vaginal thinning occurs, increasing vulnerability to inflammation and infection.9 A decline in estrogen alters the vaginal flora, which permits bacterial overgrowth, sometimes accompanied by vaginal discharge.9 These changes are also responsible for dyspareunia, which has the potential to lead to a loss of sexual interest and activity.10
Diagnosis of AV is chiefly clinical, initiating with the determination of specific symptoms including vaginal dryness, burning, pruritus, abnormal discharge, and dyspareunia.4,6 Symptoms may be more prominent in nonwhite women, those with diabetes, women who have a lower body mass index, and those who are younger at the time of menopause; more severe symptoms may appear in women who have not experienced a vaginal delivery.4,6
Upon examination, clinicians will note atrophic changes of the labia major and minor, which may include dryness and loss of subcutaneous fat; vulvar lesions and sparse pubic hair are commonly seen.4,6 There may be loss of rugae of the vaginal epithelium, with tissue appearing pale, smooth, dry, and friable; inflammation with patchy erythema and petechiae may be noted. 4,6 If clarification of diagnosis is necessary, a vaginal pH level >5.0 is indicative of AV. 4,6 Diagnosis of vulvar disorders requires a detailed history, examination, and biopsy when indicated.1 Of note, while bacterial vaginosis, candidal vaginitis, and trichomonal vaginitis are uncommon among postmenopausal women, they may occur in those individuals with risk factors.9
Atrophic Urethritis and Urinary Incontinence
Lower urinary tract symptoms are common; however, it is important to acknowledge they are attributed to underlying mechanisms that vary widely.1 While urinary incontinence is particularly disabling and distressing in the elderly, women in reproductive and early postmenopausal years may also experience this condition and suffer unnecessarily.
Overactive Bladder (OAB), caused by uninhibited detrusor muscle contractions, or detrusor overactivity, is the usual cause of incontinence in the geriatric population.1 While most cases of OAB are idiopathic, neurologic conditions such as dementia, stroke, Parkinson’s disease, and multiple sclerosis amplify the condition.11 Of note, a form of OAB with impaired contractility, characterized by urgency, frequency, a weak flow rate, and urinary retention, among other signs and symptoms, may mimic stress incontinence.12
Stress Incontinence (SI) is the most common cause of incontinence in women who are in the reproductive years or early postmenopausal years.1 It is defined as urine leakage due to abrupt increases in intra-abdominal pressure on exertion, sneezing, coughing, laughing, bending, or lifting without the presence of detrusor activity.1,12,13
SI occurs largely because of complications of childbirth and the development of atrophic urethritis—thinning of the estrogen-dependent lining of the outer urethra.5,12 Typically, it is more severe in obese people secondary to pressure from abdominal contents on the top of the bladder.12 Cooper and Smith note that this type of incontinence is usually suffered in silence despite available treatment regimens.1 Pharmacists have an opportunity to guide patients regarding these symptoms and the interventions available.
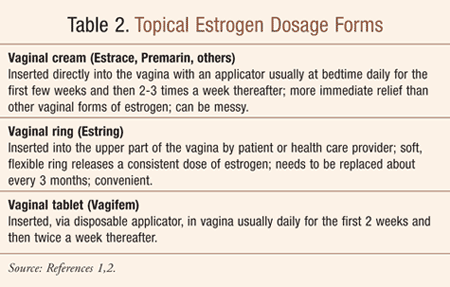
Estrogen, taken systemically and locally (Table 2), helps maintain vaginal and urethral tissue even when relatively small doses are used. Resolution of urinary symptoms is often achieved by applying a small dab of estrogen cream locally to the urethra.5 For urinary incontinence, and particularly for SI, pelvic muscle exercises (e.g., Kegel exercises–see Resources) are often effective.12, 14 Patients are instructed to specifically contract the pelvic muscles (pubococcygeus and paravaginal) instead of the abdominal, thigh, or buttock muscles.12,14 A 10-second contraction, followed by relaxation for 10 seconds, for 10 to 15 repetitions, performed three times per day is recommended; re-instruction may be necessary, and biofeedback may be useful.12 The cure rate is 10% to 25% in women under age 75 years, and improvement is seen in an additional 40% to 50%, especially in those individuals who are motivated; patients should receive written instructions and/or follow-up visits for encouragement.12
An automated version of Kegel exercises called pelvic floor electrical stimulation is available, using an electrical current that inhibits detrusor overactivity and contracts pelvic muscles.12 While it is unclear as to whether there are benefits beyond the behavioral changes alone, advantages to the electrical method are improved compliance and contraction of the correct pelvic muscles.12 For further reading on urinary incontinence beyond the scope of this column, see Reference 12.
Management: Vaginal Lubricants, Moisturizers, and Hormones
Treatment is initiated for mild symptoms with vaginal moisturizers and lubricants, a variety of which are available over the counter, to provide symptomatic relief for vaginal dryness and dyspareunia, respectively.2,4,8
If these measures are ineffective, vaginal or systemic estrogen therapy is introduced to effectively relieve vaginal dryness and itchiness and to improve vaginal elasticity.2,4 In women who have not undergone a hysterectomy, systemic estrogen therapy is accompanied by a progestin, such as medroxyprogesterone; this combination has been shown to reduce the risk of endometrial carcinoma associated with unopposed estrogen.15 A progestin may be considered with vaginal therapy as well. While both routes of administration are effective, the safety of systemic hormone therapy continues to be a concern, especially in certain subgroups of women.1,2 The National Menopause Society recommends that estrogen-progestogen therapy be prescribed at the lowest effective dose for the shortest possible time to relieve menopausal symptoms such as vasomotor instability (e.g., hot flashes, night sweats—see Reference 16), and atrophic vaginitis.1,15 For those patients who have only urogenital symptoms, including atrophic vaginitis, dyspareunia, and stress incontinence, treatment with vaginal estrogen in cream, ring, or vaginal tablet form should be used, rather than systemic estrogen (e.g., oral tablets, patches, gel, high-dose ring).15 Table 2 discusses the various topical vaginal dosage forms available.
Prevention
Sexual activity enhances blood flow to the vagina, which assists in maintaining healthy vaginal tissues.2 Furthermore, it has been suggested through observation that sexual activity can protect the vagina via stretching the vaginal tissues and possibly stimulating hormone production.17 Regular sexual activity, either with or without a partner, can decrease problems with vaginal atrophy.2 Studies indicate that testosterone, alone or with estrogen, has a beneficial effect on sexual motivation and intercourse frequency, suggesting that female sexuality is affected initially by menopause and the resulting change in hormones, more so than by aging.18
Sexuality
Sexuality and sexual behavior is a subject that is often overlooked or unaddressed by health care practitioners with regard to older adults. According to Northrup, sexual function is a complex, integrated phenomenon, reflecting the health and balance of the following: 1) the ovaries; 2) the hormones; 3) the cardiovascular system; 4) the brain; 5) the spinal cord; and 6) the peripheral nerves.5 For further reading, see Reference 5 under the chapter “Sex and Menopause: Myths and Reality,” which explores midlife changes in sexual function and sexuality, including comments on cultural inheritance and barriers. According to Cooper and Smith, since the expression of sexuality has no age limit, early and late postmenopausal women alike should be offered a full spectrum of counseling and treatment where appropriate.1
Conclusion
The hormonal environment of the female body greatly influences not only the health of the vaginal lining, but of the urethral tissues as well. Many women accept the symptoms associated with postmenopausal changes, which are often quite distressing, and believe that they are unavoidable signs of aging. Pharmacists can take a proactive role in guiding women through this time of change by providing information and guidance about therapeutic options to improve their urogenital health while helping to improve quality of life.
REFERENCES
1. Cooper TK, Smith OM. Gynecologic disorders in the elderly. In: Fillit HM, Rockwood K, Woodhouse K, eds. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. 7th ed. Philadelphia, PA: Saunders Elsevier; 2010:716-725.
2. Vaginal atrophy. Mayoclinic.com. September 17, 2010. www.mayoclinic.com/health/
3. Krychman ML. Vaginal estrogens for the treatment of dyspareunia. J Sex Med. 2011; 8(3):666-674.
4. Scudder L. Treating atrophic vaginitis. Medscape.com. Medscape Nurse. Viewpoints. Posted 07/21/2011. www.medscape.com/viewarticle/
5. Northrup C. The Wisdom of Menopause. New York, NY. Bantam Books; 2001:129-130,161-162,265-266.
6. Pearson T. Atrophic vaginitis. J Nurse Pract. 2011;7:502-505.
7. Hohenhaus MH. Vulvovaginal atrophy: a common—and commonly overlooked—problem. Med Health R I. 2011;94(5):138-140.8. Stika CS. Atrophic vaginitis. Dermatol Ther. 2010;23(5):514-522.
9. Vaginitis. MerckManual.com.
Revised April 2007.
www.merckmanuals.com/
10. Kinsey AC, Pomoroy WB, Martin CE, et al. Sexual Behaviour in the Human Female. Philadelphia, PA: WB Saunders; 1953.
11. Foon R, Toozs-Hobson P. Overactive bladder. Obstet Gynaecol Reprod Med. 2007; 17:255-360.
12. Urinary incontinence. MerckManual.com. Fully Revised August 2007/Modified March 2008. www.merckmanuals.com/
13. Dorland’s Pocket Medical Dictionary. 28th ed. Philadelphia, PA: Saunders Elsevier; 2009.
14. Kegel exercises: a how-to guide for women. Mayoclinic.com. July 10, 2010. www.mayoclinic.com/health/
15. Harvey RA, Champe PC. Pharmacology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:299-310.
16. Zagaria ME. Hot flashes and night sweats. US Pharm. 2010;35(3):20-24. www.uspharmacist.com/content/
17. Lieblum S, Bachman G, Kemmann E, et al. Vaginal atrophy in the postmenopausal woman. The importance of sexual activity and hormones. JAMA. 1983;249:2195-2198.
18. Sherwin BB, Gelfand MM. The role of androgen in the maintenance of sexual functioning in oopherectomised women. Psychosomat Med. 1987;49:397-409.
19. Beers MH, Jones TV, Berkwits M, et al, eds. The Merck Manual of Health & Aging. Whitehouse Station, NJ: Merck Research Laboratories; 2004:17,775,778,781-782,789.
To comment on this article contact rdavidson@uspharmacist.com.






