US Pharm. 2014(39):HS13-HS15.
ABSTRACT: Viral meningitis is the most common form of meningitis, affecting individuals of all ages. Enteroviruses constitute the most common host, followed by herpes simplex virus 2 (HSV-2), varicella-zoster virus, and others. The classic symptoms of viral meningitis—sudden onset of fever, stiff neck, and altered mental status—are indistinguishable from those of bacterial meningitis. Analysis of cerebrospinal fluid obtained via lumbar puncture provides a definitive diagnosis. There is no specific treatment. Complete recovery within 7 to 10 days is common, except in immunocompromised patients. In addition to symptom management, antiviral agents may be used to treat HSV-2 meningitis. The best strategy for avoiding viral meningitis is to prevent viral infection by undergoing vaccination and practicing good hygiene.
Meningitis is an inflammation of the membranes (dura, pia, and arachnoid maters) covering the brain and spinal cord. Causes of meningitis include viral, bacterial, and fungal infections, and host factors directly affect disease progression and outcome. Viral meningitis, a type of aseptic meningitis, is the most common form; it accounts for about 50% of meningitis-related hospitalizations in the United States, but most cases are self-limiting.1 In contrast, acute bacterial meningitis is a potentially fatal neurologic emergency, and survivors can have permanent neurologic complications.2 Therefore, it is critical to rapidly determine the type of meningitis during diagnosis.
Etiology and Route of Transmission
Enteroviruses (EVs) are the most common infectious agents, accounting for up to 90% of viral meningitis cases, followed by herpes simplex virus 2 (HSV-2) and other viruses.3-6
EVs: EV meningitis occurs sporadically, with outbreaks commonly occurring in the summer and early fall (the enteroviral season). The reported incidence may be underestimated, since most cases are mild and do not require hospital admission.7 Within the EV family, coxsackieviruses and echoviruses are responsible for the majority of cases.8 Infants, young children (<5 years), and elderly persons who interact or reside in group settings are more susceptible, but the incidence in children tends to decrease with age.3,9 Transmission is mediated primarily via the fecal-oral route or through respiratory droplets and fomites (inanimate objects or substances that can carry infectious organisms, such as utensils).3 EV meningitis can cause significant morbidity with respect to hospitalization and duration of impaired activity.4 Severe complications are uncommon, except in immunocompromised patients.7 Although rare, cases of concomitant EV meningitis and bacterial meningitis have been reported.10,11
Meningitis can occur as an uncommon neurologic complication of enterovirus 71 (EV71), a common cause of hand-foot-mouth disease in children. EV71 outbreaks occur periodically in the Asian Pacific countries and rarely in the U.S.12,13
Herpesviruses: Of the three herpesviruses that can remain latent in the host for a lifetime, herpes simplex virus 1 (HSV-1) is more associated with encephalitis, whereas HSV-2 and varicella-zoster virus (VZV) cause meningitis.7,14 These viruses cause primary infection of mucocutaneous surfaces, establish latency in the peripheral sensory ganglia through retrograde transportation, and reactivate periodically with anterograde transmission to the nerve endings and mucocutaneous surfaces.14
HSV-2 meningitis, which is far more common than VZV meningitis, may develop during or after a primary genital infection, which is acquired mainly through sexual contact.14-16 Among patients with primary genital herpes, 36% of women and 13% of men have been reported to develop meningitis as a complication.14,17 HSV-2 meningitis can also occur without any genital herpes symptoms.18 Once a person develops HSV-2 meningitis, there is a 19% to 42% chance that meningitis will recur over his or her lifetime.19,20 A 56-year interval between the initial genital herpes infection and the first recurrent HSV-2 meningitis episode was reported in a 78-year-old patient.16 Recurrent benign lymphocytic meningitis (RBLM), also known as Mollaret meningitis, is a rare disease most frequently caused by HSV-2. RBLM is characterized by three to 10 episodes of benign meningitis that last for 2 to 5 days before spontaneous recovery.21
VZV, which causes chickenpox (varicella), may reactivate to cause shingles (herpes zoster). Meningitis is a rare complication of primary infection and is more common during reactivation. VZV meningitis also may occur without cutaneous symptoms. In a study involving 21 patients with VZV meningitis, more than 50% had no skin lesions.22 Transmission occurs mainly through direct or indirect contact with the saliva, sputum, or mucus of an infected person.3
Other HSVs associated with meningitis are cytomegalovirus (CMV), Epstein-Barr virus (EBV), and human herpesvirus 6. Central nervous system infections from CMV are seen mostly in immunocompromised patients.3,8
Mumps and Measles: Mumps, a childhood infection characterized by swelling of the parotid salivary gland, is caused by the mumps virus. Meningitis was a common complication before the introduction of immunization. Mumps can be spread through direct or indirect contact with the saliva or mucus of an infected person.3 Despite routine vaccination, mumps outbreaks occurred in the Midwest in 2006, affecting mostly young adults.23 Morbillivirus, which is responsible for measles, in rare cases can cause meningitis.3
HIV and Other Viruses: HIV may be a cause of viral meningitis during seroconversion (presence of anti-HIV antibodies in blood). The meningitis may persist in a small number of cases, and in some instances it may be complicated by cranial neuropathies.7
Arthropod-borne viruses (arboviruses) such as West Nile virus account for a few cases of meningitis each year, usually during the summer and early fall, when insect populations are high.24 Lymphocytic choriomeningitis virus (LCMV), which is spread by rodents, in rare cases may cause meningitis.25 Contact with the blood, saliva, urine, droppings, or nesting materials of infected rodents can lead to transmission.
Pathogenesis
Viruses enter the brain by two major routes: hematogenous (carried by blood) or neural (carried by nerves). Most EV infections start in the intestine, after the individual swallows the initial inoculum, and spread to other tissues and the blood. Brain penetration is mediated by the hematogenous pathway. HSVs (HSV-1, HSV-2, VZV) enter the brain mainly via the peripheral and cranial nerves. Once inside the brain, the virus spreads through the subarachnoid space in cerebrospinal fluid (CSF), and its rapid replication can overcome the host defenses. Inflammatory WBCs such as lymphocytes accumulate and target the virus. Consequently, inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α are released. Inflammatory responses can increase the permeability of the blood-brain barrier, allowing the entrance of circulating immunoglobulins.7,26
Symptoms
The classic triad of viral meningitis symptoms consists of sudden-onset (occurring hours to 1-2 days after infection) fever, stiff neck (nuchal rigidity), and altered mental status. Other symptoms include nausea, vomiting, and sensitivity to light.3,8 Irritability, refusal to eat, and rash are possible symptoms in infants and young children.27
EV meningitis may be accompanied by symptoms including localized vesicles, herpangina, and generalized maculopapular rash.8 Seizures or an abrupt deterioration in mental status may indicate progression to meningoencephalitis.28 Back, buttock, perineal, or lower-extremity pain, urinary retention, constipation, paresthesias, and motor weakness are complications of HSV-2 meningitis.14,17 In mumps meningitis, swelling of the salivary gland is observed in about 50% of cases.7
Most symptoms of viral meningitis last from 7 to 10 days, and patients with a normal immune system usually recover completely.3
Diagnosis
Because the symptoms of viral meningitis are indistinguishable from those of bacterial meningitis, hospitalization, empirical prescription of antibiotics, and lumbar puncture are necessary for exclusion of bacterial meningitis. Identification of a viral cause can reduce antibiotic use and shorten hospital stays.6,8
Lumbar Puncture for CSF Analysis: A definitive diagnosis is obtained by analyzing CSF collected via lumbar puncture. Analysis includes WBC count; differential, protein, and glucose levels; microscopy with Gram stain; and culture (TABLE 1). In contrast to bacterial meningitis, the opening pressure and glucose level are usually normal in viral meningitis, and the protein level is only slightly elevated. While bacterial meningitis is characterized by polymorphonuclear leukocyte (PMNL) predominance, mononuclear lymphocyte predominance is common in viral meningitis.8,29,30 However, PMNL predominance and persistence beyond 24 hours have been observed in children with viral meningitis.31 Therefore, PMNL predominance cannot be regarded as a sole criterion for diagnosis.
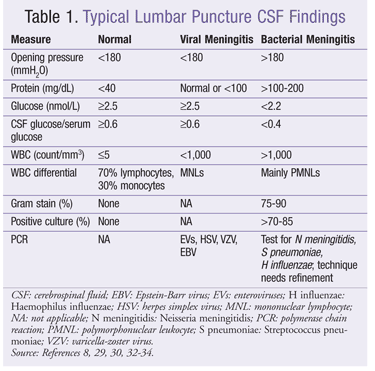
Despite its high cost, polymerase chain reaction (PCR) can rapidly and accurately detect EV, HSV, VZV, and EBV.32-34 A positive PCR test can prevent unnecessary hospital admission in a patient with mild symptoms.30 EV meningitis may be difficult to differentiate from partially treated bacterial meningitis because antibiotic treatment can lead to unreliable Gram stain results. Therefore, many patients with viral meningitis are treated with empirical antibiotics in the hospital until the PCR test results are known.35
Physical Examination: Kernig’s and Brudzinski’s signs pertain to inflammation of the meninges and nerve endings.36 At one time, both were used to diagnose meningitis. Kernig’s sign is the inability to straighten the leg when the hips are flexed to a 90-degree angle; Brudzinski’s sign is the spontaneous flexion of the hips and knees during attempted flexion of the neck. Because of individual patients’ variations in sensitivity and specificity, these methods should be used only to supplement diagnosis.
Treatment
No specific treatment is recommended for viral meningitis, and most patients, except for those who are immunocompromised, recover completely on their own within 7 to 10 days.3 The main therapeutic strategy is symptom management, including prevention of dehydration, reduction of body temperature with antipyretics, and alleviation of pain with analgesics.4
Variable antiviral treatment, including agents such as acyclovir and valacyclovir, has been used for uncomplicated HSV-2 meningitis.37,38 Suppressive prophylactic therapy with acyclovir, famciclovir, and valacyclovir may help prevent the recurrence of HSV-2 RBLM.14 Patients with RBLM should be counseled regarding genital herpes and its transmission.21
Long-Term Sequelae
Recovery from viral meningitis is usually complete, although some patients experience short-term memory loss, cognitive impairment, and sleep disturbances after recovery.39-41 Other sequelae include fatigue and depression, which may be related to prolonged convalescence and previous psychiatric history.42 Infants (<1 year) with meningitis may later exhibit subtle neurodevelopmental setbacks, such as language problems.43
Compared with other forms of viral meningitis, HSV-2 meningitis causes more neurologic complications that are likely to resolve after 6 months.19 Long-term neurologic sequelae may occur in immunocompromised patients. Prompt treatment with acyclovir and valacyclovir may be helpful in preventing sequelae.38
Prevention
The best strategy for preventing viral meningitis is to prevent viral infection. Vaccination against measles and mumps (MMR vaccine) and chickenpox (varicella-zoster vaccine) effectively protects children against viral meningitis.3
Adherence to good-hygiene practices—thorough hand-washing, especially after changing diapers, using the toilet, coughing, or blowing one’s nose—is important for preventing transmission. Other key practices include cleaning contaminated surfaces and avoiding the sharing of utensils.
To prevent arbovirus infection, protection from mosquito bites by wearing appropriate clothing and using insect repellent and bed nets should be exercised, especially while traveling.44
To reduce the risk of LCMV infection, pregnant women should avoid exposure to rats, house mice, and rodents such as hamsters and guinea pigs. Any person who develops fever or other symptoms after contact with a rodent should seek medical attention.25
Conclusion
Viral meningitis can affect individuals of any age. Pharmacists can educate patients about the signs of meningitis and the importance of determining the type of meningitis in a hospital setting to avoid treatment delays in case the meningitis is bacterial. Pharmacists should also inform patients that preventive measures, such as routine vaccination and proper hygiene, can significantly reduce the risk of viral meningitis.
REFERENCES
1. Holmquist L, Russo CA, Elixhauser A. Meningitis-Related Hospitalizations in the United States, 2006. HCUP Statistical Brief #57. Rockville, MD: Agency for Healthcare Research and Quality; July 2008.
2. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired
bacterial meningitis: risk stratification for adverse clinical outcome
and effect of antibiotic timing. Ann Intern Med. 1998;129:862-869.
3. CDC. Viral meningitis. www.cdc.gov/meningitis/viral.html. Accessed January 10, 2014.
4. Rotbart HA, Brennan PJ, Fife KH, et al. Enterovirus meningitis in adults. Clin Infect Dis. 1998;27:896-898.
5. Kupila L, Vuorinen T, Vainionpää R, et al. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology. 2006;66:75-80.
6. Ramers C, Billman G, Hartin M, et al. Impact of a diagnostic
cerebrospinal fluid enterovirus polymerase chain reaction test on
patient management. JAMA. 2000;283:2680-2685.
7. Chadwick DR. Viral meningitis. Br Med Bull. 2005;75-76:1-14.
8. Logan SA, MacMahon E. Viral meningitis. BMJ. 2008;336:36-40.
9. Strikas RA, Anderson LJ, Parker RA. Temporal and geographic
patterns of isolates of nonpolio enteroviruses in the United States,
1970-1983. J Infect Dis. 1986;153:346-351.
10. Sferra TJ, Pacini DL. Simultaneous recovery of bacterial and viral pathogens from cerebrospinal fluid. Pediatr Infect Dis J. 1988;7:552-556.
11. Basmaci R, Mariani P, Delacroix G, et al. Enteroviral meningitis does not exclude concurrent bacterial meningitis. J Clin Microbiol. 2011;49:3442-3443.
12. AbuBakar S, Sam IC, Yusof J, et al. Enterovirus 71 outbreak, Brunei. Emerg Infect Dis. 2009;15:79-82.
13. Pérez-Vélez CM, Anderson MS, Robinson CC, et al. Outbreak of
neurologic enterovirus type 71 disease: a diagnostic challenge. Clin Infect Dis. 2007;45:950-957.
14. Berger JR, Houff S. Neurological complications of herpes simplex virus type 2 infection. Arch Neurol. 2008;65:596-600.
15. Simko JP, Caliendo AM, Hogle K, Versalovic J. Differences in
laboratory findings for cerebrospinal fluid specimens obtained from
patients with meningitis or encephalitis due to herpes simplex virus
(HSV) documented by detection of HSV DNA. Clin Infect Dis. 2002;35:414-419.
16. Davis LE, Guerre J, Gerstein WH. Recurrent herpes simplex virus type 2 meningitis in elderly persons. Arch Neurol. 2010;67:759-760.
17. Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex
virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958-972.
18. O’Sullivan CE, Aksamit AJ, Harrington JR, et al. Clinical
spectrum and laboratory characteristics associated with detection of
herpes simplex virus DNA in cerebrospinal fluid. Mayo Clin Proc. 2003;78:1347-1352.
19. Bergström T, Vahlne A, Alestig K, et al. Primary and recurrent herpes simplex virus type 2-induced meningitis. J Infect Dis. 1990;162:322-330.
20. Afonso N, Gunasena S, Galla K, et al. Appropriate use of
polymerase chain reaction for detection of herpes simplex virus 2 in
cerebrospinal fluid of patients at an inner-city hospital. Diagn Microbiol Infect Dis. 2007;57:309-313.
21. Shalabi M, Whitley RJ. Recurrent benign lymphocytic meningitis. Clin Infect Dis. 2006;43:1194-1197.
22. Echevarría JM, Casas I, Tenorio A, et al. Detection of
varicella-zoster virus-specific DNA sequences in cerebrospinal fluid
from patients with acute aseptic meningitis and no cutaneous lesions. J Med Virol. 1994;43:331-335.
23. Hviid A, Rubin S, Mühlemann K. Mumps. Lancet. 2008;371:932-944.
24. CDC. West Nile virus and other arboviral diseases—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:513-517.
25. CDC. Lymphocytic choriomeningitis virus (LCMV) and pregnancy.
www.cdc.gov/pregnancy/infections-lcmv.html. Accessed January 10, 2014.
26. Irani D. Aseptic meningitis and viral myelitis. Neurol Clin. 2008;26:635-655.
27. Dagan R, Jenista JA, Menegus MA. Association of clinical
presentation, laboratory findings, and virus serotypes with the presence
of meningitis in hospitalized infants with enterovirus infection. J Pediatr. 1988;113:975-978.
28. Sawyer MH, Rotbart H. Viral meningitis and aseptic meningitis syndrome. In: Scheld WM, Whitley RJ, Marra CM, eds. Infections of the Central Nervous System. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004:75-93.
29. Tacon CL, Flower O. Diagnosis and management of bacterial meningitis in the paediatric population: a review. Emerg Med Int. 2012;2012:320309.
30. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68:1103-1108.
31. Negrini B, Kelleher KJ, Wald ER. Cerebrospinal fluid findings in aseptic versus bacterial meningitis. Pediatrics. 2000;105:316-319.
32. Smalling TW, Sefers SE, Li H, Tang YW. Molecular approaches to
detecting herpes simplex virus and enteroviruses in the central nervous
system. J Clin Microbiol. 2002;40:2317-2322.
33. Iten A, Chatelard P, Vuadens P, et al. Impact of cerebrospinal
fluid PCR on the management of HIV-infected patients with
varicella-zoster virus infection of the central nervous system. J Neurovirol.1999;5:172-180.
34. Weinberg A, Li S, Palmer M, Tyler KL. Quantitative CSF PCR in Epstein-Barr virus infections of the central nervous system. Ann Neurol. 2002;52:543-548.
35. Nolte FS, Rogers BB, Tang YW, et al. Evaluation of a rapid and
completely automated real-time reverse transcriptase PCR assay for
diagnosis of enteroviral meningitis. J Clin Microbiol. 2011;49:528-533.
36. Ward MA, Greenwood TM, Kumar DR, et al. Josef Brudzinski and Vladimir Mikhailovich Kernig: signs for diagnosing meningitis. Clin Med Res. 2010;8:13-17.
37. Landry ML, Greenwold J, Vikram HR. Herpes simplex type-2 meningitis: presentation and lack of standardized therapy. Am J Med. 2009;122:688-691.
38. Miller S, Mateen FJ, Aksamit AJ Jr. Herpes simplex virus 2 meningitis: a retrospective cohort study. J Neurovirol. 2013;19:166-171.
39. Sittinger H, Müller M, Schweizer I, Merkelbach S. Mild cognitive impairment after viral meningitis in adults. J Neurol. 2002;249:554-560.
40. Schmidt H, Heimann B, Djukic M, et al. Neuropsychological sequelae of bacterial and viral meningitis. Brain. 2006;129:333-345.
41. Schmidt H, Cohrs S, Heinemann T, et al. Sleep disorders are long-term sequelae of both bacterial and viral meningitis. J Neurol Neurosurg Psychiatry. 2006;77:554-558.
42. Hotopf M, Noah N, Wessely S. Chronic fatigue and minor psychiatric morbidity after viral meningitis: a controlled study. J Neurol Neurosurg Psychiatry. 1996;60:504-509.
43. Wilfert CM, Thompson RJ Jr, Sunder TR, et al. Longitudinal
assessment of children with enteroviral meningitis during the first
three months of life. Pediatrics. 1981;67:811-815.
44. Nasci RS, Zielinski-Gutierrez E, Wirtz RA, Brogdon WG. Protection
against mosquitoes, ticks, & other insects & arthropods.
wwwnc.cdc.gov/travel/yellowbook/2014/chapter-2-the-pre-travel-consultation/protection-against-mosquitoes-ticks-and-other-insects-and-arthropods.
Accessed March 10, 2014.
To comment on this article, contactrdavidson@uspharmacist.com.





