US Pharm. 2019;44(9):5-9.
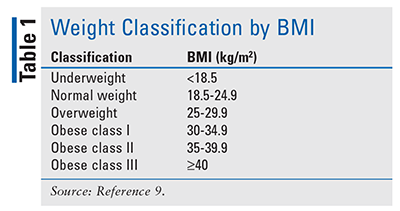
Obesity is defined by the World Health Organization as “an abnormal or excessive fat accumulation that presents a risk to health,” and it is further classified in adults as a BMI of 30 or higher (TABLE 1).1 Obesity affects approximately 93.3 million U.S. adults and 13.7 million children and adolescents.2,3 The prevalence of obesity in the U.S. has been increasing since the 1980s. In 1985, no state had an obesity rate above 15%; currently, more than 20% of adults in all states are obese, with seven states having rates over 35%.4,5 It is projected that if this trend continues, up to 51% of the U.S. adult population will be obese by 2030.6 The increasing number of obese adults and children has become a public-health problem that is associated with greater morbidity and mortality and decreased quality of life. Over time, obesity can lead to other health consequences, such as diabetes, heart disease, stroke, dyslipidemia, hypertension, and cancer. It is estimated that obesity contributes to 112,000 preventable deaths annually.7
One obstacle to managing obesity is the difficulty of maintaining long-term weight loss. However, by engaging in regular physical activity and making informed food choices, individuals can attain and maintain a healthy weight. Three main goals for people who want to lose weight are to prevent further weight gain, reduce body weight, and maintain lower body weight over time. A reasonable initial weight-loss goal is 10% of baseline body weight over a 6-month period, losing no more than 2 lb per week.8 Even modest weight loss can produce health benefits such as improvements in blood cholesterol and blood sugar. Pharmacists can play a role in helping patients achieve their weight-loss goals by educating them about healthful lifestyle changes and the risks and benefits associated with OTC supplements.
Lifestyle Modification
Lifestyle modification is crucial to the success of any weight-loss management plan. Lifestyle therapy should include three components: a reduced-calorie healthful meal plan, physical activity, and behavior therapy. Any drug therapy for overweight and obesity should be used in conjunction with lifestyle modifications, not as monotherapy.9
Diet: Reduction of calorie intake should be considered the cornerstone of any weight-loss and weight-maintenance plan. Three options are available: a 1,200 to 1,500 kcal/day diet for women or a 1,500 to 1,800 kcal/day diet for men; a diet that is 500 to 750 kcal less than the patient’s usual diet; or an evidence-based diet that restricts certain food types. Increased consumption of nutrient-poor added fats, sugar, salt, and refined grains contributes to obesity. A healthful diet should include a variety of fruits, vegetables, whole grains, and high-quality protein. Drinking more water and controlling portion sizes are two other behaviors that can promote weight loss.8-11
Exercise: Health-based weight loss requires a lifestyle change that includes daily physical activity. In addition to promoting weight loss, exercise can lower the risk of many disease states and aid in stress management. It is recommended to start with moderate-intensity aerobic exercise, such as walking, running, cycling, or swimming, for at least 150 minutes weekly over 3 to 5 sessions per week. The patient should increase the duration (number of minutes per session) and frequency (number of days per week) of moderate-intensity activity before increasing the intensity. Patients with a low level of physical activity should start with light activity and slowly integrate moderate-intensity activities. A higher level of physical activity—approximately 200 to 300 minutes per week—is recommended to maintain weight loss and minimize weight regain for the long term. Resistance training should also be incorporated to help promote fat loss while preserving lean mass. The key to exercise and weight loss is to be physically active daily and consistently. Activities should be individualized according to patient preference, taking into account any health-related or physical limitations.8-10,12
Behavior Therapy: For reduced-calorie diets and exercise to be successful for weight loss, the patient must adhere to these regimens, resulting in a change of lifestyle. To achieve this end, behavioral interventions should be incorporated. Such interventions may include self-monitoring of weight, food intake, and exercise; personal goal setting; education via group meetings or virtual meetings; stress reduction; motivational interviewing; counseling; and problem-solving strategies. The goal of behavior therapy is to help the patient increase his or her capacity for self-control by changing habits and maintaining the new habits.8-10,13
Orlistat
Orlistat (brand name: alli) is the only OTC medication that is FDA approved for weight loss in conjunction with reduced calorie intake. The OTC version of orlistat (60 mg) is indicated for overweight adults aged 18 years or older in conjunction with a reduced-calorie, low-fat diet.14 Orlistat does not act systemically; instead, it exerts its therapeutic activity in the lumen of the stomach and small intestine by inhibiting gastric and pancreatic lipases that hydrolyze triglycerides into free fatty acids and monoglycerides. This restricts the intestine’s ability to absorb triglycerides, which are excreted fecally instead, thus inhibiting absorption of dietary fats by approximately 30%.15 In a 16-week randomized, controlled study, orlistat 60 mg resulted in significant weight loss compared with placebo (3.05 kg vs. 1.9 kg; P <.001) in mildly to moderately overweight adults.16 Another study evaluated the ability of orlistat 60 mg to produce a change in visceral adipose tissue in overweight patients.17 After 24 weeks, orlistat demonstrated a significant decrease in visceral adipose tissue versus placebo (–15.7% vs. –9.4%; P <.05). In addition, there was a trend toward a greater reduction in liver fat (which is independently linked to dyslipidemia and insulin resistance) and intermuscular adipose tissue (which is associated with metabolic abnormalities related to muscle and glucose metabolism).17 These findings suggest that orlistat 60 mg, along with a reduced-calorie, low-fat diet, may be an effective weight-loss tool for reducing metabolic risk factors associated with upper-body adiposity.
Because the absorption of orlistat is minimal, there are very few systemic effects. Common adverse effects are gastrointestinal (GI) and are caused by the increased amount of fat in the GI tract. These effects may include flatulence with discharge, fecal urgency, oily spotting, fatty or oily stool, abdominal pain or discomfort, and increased defecation. These symptoms seem to improve over time, usually lasting no longer than 4 weeks. Rarely, severe liver injury has been reported with orlistat use, but a causal relationship has not been established. Patients should be instructed to stop taking orlistat and to speak with their healthcare provider if they develop signs and symptoms of liver injury, including itching, yellowed eyes or skin, dark urine, fever, right-upper-quadrant abdominal pain, or loss of appetite.15,18
Owing to orlistat’s mechanism of action, a decrease in the absorption of fat-soluble vitamins (vitamin A, D, E, and K) has been noted. This occurs because orlistat inhibits the lipase that breaks down fat-soluble vitamins into absorbable components. It is recommended that the patient take a multivitamin containing fat-soluble vitamins in order to ensure adequate intake. Multivitamin administration should be separated from orlistat by at least 2 hours or should occur at bedtime. Orlistat was found to decrease serum concentrations of amiodarone, cyclosporine, levothyroxine, and antiepileptic medications. It is recommended to separate doses of orlistat from cyclosporine by 3 hours and from levothyroxine by 4 hours. Patients who are taking antiepileptic medications concurrently should be monitored closely for any changes in frequency or severity of convulsions. Because of decreased vitamin K absorption, warfarin concentrations may also be affected, resulting in an increased international normalized ratio; patients should be monitored closely when this vitamin is taken with orlistat. It is also important to tell patients with diabetes who are taking orlistat that weight loss may increase the risk of lower blood sugar and that they should watch for signs of low blood sugar, such as dizziness, headache, feeling weak or shaky, sweatiness, or a fast heartbeat. Orlistat is contraindicated in patients who are pregnant, have chronic malabsorption syndrome or cholestasis, or have known orlistat hypersensitivity.15
The recommended dosage of orlistat OTC is 60 mg three times per day before main meals containing between 12 and 18 g of fat or up to 1 hour afterward; the drug blocks up to 25% of fat absorption. If the patient skips a meal, the orlistat dose should also be skipped. Orlistat acts on each individual meal and is dependent on the types of food the patient eats and the patient’s GI transit time. In a clinical study, weight loss was observed within 2 weeks of therapy initiation and continued for 6 to 12 months.15 Regardless of results, it is important for patients to continue to be physically active and maintain a healthy diet even after discontinuing orlistat.15
Dietary Supplements
The use of dietary supplements is very common; almost 34% of people attempting to lose weight have tried a dietary weight-loss supplement.19 Many patients believe that these supplements are safe and effective because they are considered “natural” and are readily available in many retail settings. Some common therapies include caffeine, chromium, cinnamon, garcinia, and green tea extract. Under the Federal Food, Drug, and Cosmetics Act, dietary supplements do not need FDA approval prior to marketing. In the past, the FDA has received many reports of harm associated with the use of weight-loss products, withdrawing many weight-loss ingredients of nonprescription products from the market, including ephedra, phenylpropanolamine, aloe, and cascara sagrada. In 2014 alone, the FDA issued more than 30 public notifications and recalled seven tainted weight-loss products.20
Much of the evidence for weight-loss supplements is conflicting at best. Many of these products do not offer impressive weight-loss benefits, and most are associated with harmful side effects and interactions. Supervision is extremely important in order to protect the patient; however, many patients do not disclose or discuss weight-loss supplement use with health providers.9,21
The Pharmacist’s Role
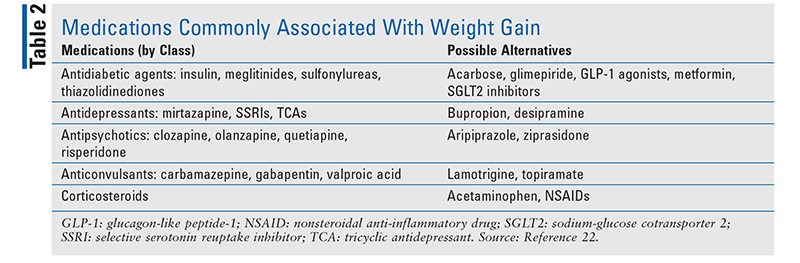
Pharmacists can have a beneficial effect overall on weight loss and the obesity epidemic. Regardless of the practice setting, pharmacists are in an ideal position to help patients on their journey to a healthy weight. They can provide education and information about appropriate weight-loss programs and healthy eating habits. Pharmacists can refer patients who may require further evaluation or additional services, such as nutritional counseling or behavior therapy. In addition, pharmacists can determine whether a patient’s weight gain potentially may be caused by a prescription medication (TABLE 2).22,23 Importantly, pharmacists can inquire about supplement use and discourage inappropriate use of OTC weight-loss supplements.
Conclusion
Obesity has reached epidemic levels nationwide. It is associated with increased risk of many other diseases, including diabetes, coronary heart disease, and certain cancers. Obesity accounts for 18% of deaths among Americans aged 40 to 85 years.24 Lifestyle modifications, including a reduced-calorie diet, increased physical activity, and behavior therapy, should be the cornerstone of any weight-loss program. Drug therapy should always be used in conjunction with lifestyle changes. Orlistat provides an FDA-approved OTC option for weight loss. Patients should be educated about the importance of continued behavior modification if orlistat is added.

How can weight be managed?
You can manage your weight by changing your diet, exercising, or taking medications. Lifestyle changes are generally the most beneficial management behaviors because they can help you maintain long-term weight loss and keep excess weight off. It is important to eat more healthful foods, such as fruits, vegetables, and whole grains, and to avoid a diet high in saturated fats and trans fats. Also avoid sweet drinks and add more water to your diet. Women should consume no more than 1,500 calories per day and men no more than 1,800 calories per day. When exercising, aim for 150 minutes per week of moderate-intensity exercise over 3 to 5 days per week. There is one FDA-approved over-the counter (OTC) weight-loss product, orlistat, that can be helpful when used together with a reduced-calorie diet. Supplements touted for weight loss are also available OTC, but they are not FDA approved. It is important to always consult a healthcare provider before starting any new medication or supplement.
What is orlistat?
Orlistat (brand name: alli) is an FDA-approved OTC medication that can help overweight adults lose weight. Orlistat works by blocking fat from being absorbed into the body after it is eaten during a meal. Approximately 5 to 10 pounds may be lost during the first 6 months of orlistat use.
How do you take orlistat?
One orlistat capsule is taken with a meal, or within 1 hour of each meal containing fat, three times per day (breakfast, lunch, and dinner). If you skip a meal, do not take orlistat. No more than three capsules of orlistat should be taken in a single day. A multivitamin is recommended when orlistat is used, but it should be taken at least 2 hours after orlistat or at bedtime. Continue to be physically active and control your diet during and after taking orlistat to help maintain the weight loss.
What are the side effects of orlistat?
Some common side effects include flatulence, frequent bowel movements, soft stool, oily rectal leakage, and abdominal pain. Speak with a healthcare provider if any of these side effects become severe or troublesome. If you experience any serious side effects, such as hives, rash, skin blistering, right-sided upper stomach or abdominal pain, pain radiating toward the back, or fever, stop taking the medication and immediately contact a healthcare provider.
REFERENCES
1. World Health Organization. Obesity. www.who.int/topics/obesity/en. Accessed August 10, 2019.
2. CDC. Adult obesity facts. www.cdc.gov/obesity/data/adult.html. Accessed August 10, 2019.
3. CDC. Childhood obesity facts. www.cdc.gov/obesity/data/childhood.html. Accessed August 10, 2019.
4. Henry TA. Adult obesity rates rise in 6 states, exceed 35% in 7. www.ama-assn.org/delivering-care/public-health/adult-obesity-rates-rise-6-states-exceed-35-7. Accessed August 10, 2019.
5. CDC. Adult obesity prevalence maps. www.cdc.gov/obesity/data/prevalence-maps.html. Accessed August 10, 2019.
6. Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563-570.
7. Benjamin RM. Office of the Surgeon General (US). The Surgeon General’s vision for a healthy and fit nation. Background on obesity. Rockville, MD: Office of the Surgeon General; 2010. www.ncbi.nlm.nih.gov/books/NBK44656. Accessed August 10, 2019.
8. NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Heart, Lung, and Blood Institute; 1998. NIH Publication No. 98-4083.
9. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203.
10. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25 suppl 2):S102-S138.
11. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6(3):e73.
12. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56(4):441-447.
13. Adachi Y. Behavior therapy for obesity. JMAJ. 2005;48(11):539-544.
14. alli is the only OTC weight loss aid approved by the FDA. www.myalli.com/about/product-use. Accessed August 10, 2019.
15. Xenical (orlistat) package insert. Montgomery, AL: H2-Pharma, LLC; August 2017.
16. Anderson JW, Schwartz SM, Hauptman J, et al. Low-dose orlistat effects on body weight of mildly to moderately overweight individuals: a 16 week, double-blind, placebo-controlled trial. Ann Pharmacother. 2006;40(10):1717-1723.
17. Smith SR, Stenlof KS, Greenway FL, et al. Orlistat 60 mg reduces visceral adipose tissue: a 24-week randomized, placebo-controlled, multicenter trial. Obesity (Silver Spring). 2011;19(9):1796-1803.
18. alli. Frequently asked questions. www.myalli.com/alli-faq. Accessed August 10, 2019.
19. Pillitteri JL, Shiffman S, Rohay JM, et al. Use of dietary supplements for weight loss in the United States: results of a national survey. Obesity (Silver Spring). 2008;16(4):790-796.
20. FDA. Beware of products promising miracle weight loss. www.fda.gov/consumers/consumer-updates/beware-products-promising-miracle-weight-loss. Accessed August 10, 2019.
21. Blanck HM, Serdula MK, Gillespie C, et al. Use of nonprescription dietary supplements for weight loss is common among Americans. J Am Diet Assoc. 2007;107(3):441-447.
22. Malone M. Medications associated with weight gain. Ann Pharmacother. 2005;39(12):2046-2055.
23. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94(5):361-368.
24. Masters RK, Reither EN, Powers DA, et al. The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103(10):1895-1901.
To comment on this article, contact rdavidson@uspharmacist.com.





