US Pharm. 2019:44(11)18-22.
Influenza, more commonly referred to as the flu, has plagued humanity numerous times and has appeared throughout history under many different names, including the Spanish flu of 1918 or the Swine flu of 2009. Fortunately, vaccines have been developed to combat these viruses and have saved countless lives. Despite influenza vaccines being over half a century old, influenza is still the leading cause of death by a vaccine-preventable disease in the United States; together with pneumonia, influenza is the eighth leading cause of death in the U.S.1 For the 2017–2018 season, the CDC estimated almost 49 million people were infected with the flu; this is the highest number seen since the 2009 H1N1 pandemic, when approximately 60 million people were infected. Overall, there were 959,000 hospitalizations and 79,400 deaths attributed to influenza during the 2017–2018 season.2
The flu can affect people of all ages, but the highest rates of complications and hospitalizations are most often seen in those aged 65 years or older, young children, and persons with certain underlying medical conditions. In the 2017–2018 season, there were 186 influenza-associated pediatric deaths, and older adults accounted for 90% of the influenza deaths reported. Despite the widespread availability of a vaccine, many choose not to be vaccinated; approximately 80% of these deaths occurred in children who had not received a flu vaccination.3 People may choose not to vaccinate because of the uncertainties and controversies regarding the influenza vaccine, including how the vaccine works, the most appropriate time to receive the vaccine, concerns about harmful long-term side effects, and the vaccine’s ingredients.
Influenza Vaccines
Influenza vaccines induce neutralizing antibodies against the viral surface proteins hemagglutinin (HA) and neuraminidase (NA), but the vaccine is quantified only for its HA content, and vaccine efficacy is determined by the presence of adequate HA inhibition.4,5 The influenza vaccine is available as either killed or weakened versions of the virus, also known as inactivated influenza vaccine (IIV) or live attenuated influenza vaccine (LAIV), respectively. The IIVs have been available since the 1940s and are the most commonly used formulations.
Currently, there are three different influenza technologies in use that are approved by the FDA to produce influenza vaccines. These include egg-based, cell-cultured, and recombinant technologies. Egg-based IIVs are prepared from inactivated and detergent-solubilized virion particles containing HA and NA proteins prepared in embryonated chicken eggs. The live attenuated vaccine is prepared from attenuated, less virulent virus strains.6-8 Cell-cultured vaccines are produced by growing the influenza virus in animal cells, allowing for a faster manufacturing process. Although hen’s eggs are not used, trace amounts of egg protein may be found in these vaccines.9,10 The recombinant flu vaccine is an alternative method that isolates the HA gene and proteins in order to obtain an immunogenic response without the use of chicken eggs. This vaccine is produced in insect cell cultures and contains three times the amount of HA compared with the standard-dose preparation. It is currently the only available influenza vaccine that is completely egg-free.8,11
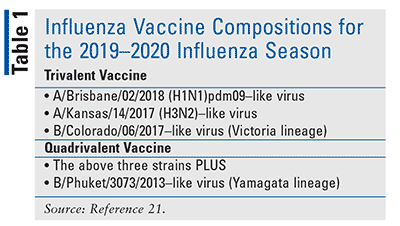
Two types of influenza viruses are included in the influenza vaccine: human influenza A virus and influenza B virus. Vaccine composition is reviewed annually and updated as needed based on the circulating viruses, extent to which those viruses are spreading, and how good the response was to the previous year’s vaccine. The World Health Organization makes recommendations about the specific viruses to include, and the FDA makes the final decision as to which strains will be included in the upcoming season’s influenza vaccines for administration in the U.S. The conventional trivalent vaccines protect against influenza A viruses H1N1 and H3N2 and an influenza B virus (either Victoria or Yamagata strain). The quadrivalent vaccines offer the same coverage as the trivalent, with the addition of another B strain virus, increasing the likelihood for adequate protection.12,13
For those persons aged 65 years and older, there are currently two influenza vaccines designed to overcome the waning immune response found in this patient population, high-dose IIV (HD-IIV) and adjuvanted IIV (aIIV). Both formulations are available only as a trivalent vaccine. The HD-IIV contains four times the HA-antigen compared with standard influenza vaccines, providing a better immune response and reducing clinical outcomes associated with influenza infection in this patient population.14-16 When compared with patients who received standard-dose trivalent influenza vaccines, HD-IIV was shown to be 24% more effective in preventing laboratory-confirmed influenza.16 Unlike HD-IIV, the aIIV is a standard-dose vaccine, but it is formulated with the adjuvant MF59.17 This formulation has been used in Europe since 1997 and was approved for use in the U.S. beginning with the 2016–2017 influenza season. It has also been shown to be effective in reducing influenza and influenza-related outcomes in this patient population.18,19 To date, there are no studies directly comparing HD-IIV with aIIV; however, there is a prospective, randomized, blinded trial designed for the 2017–2018 and 2018–2019 influenza seasons to compare the safety and immunogenicity of these two vaccines.20
The 2019–2020 influenza vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) have remained mostly the same, with the exception of adding the LAIV to the immunization schedule. The FDA has made label changes to Afluria Quadrivalent and Fluzone Quadrivalent; Afluria Quadrivalent can now be given to children aged 6 months and older, while Fluzone Quadrivalent is now approved to be given at a dose of 0.5 mL for children aged 6 months to 35 months. Lastly, changes have been made to the composition of the trivalent and quadrivalent vaccines for the 2019–2020 influenza season (Table 1).21,22
Efficacy
Influenza vaccines’ effectiveness varies every year and depends on a patient’s characteristics, such as age and health status, and the similarity, or match, of the vaccine to the circulating strains in the community. When the vaccine matches the viruses in circulation, the risk is reduced 40% to 60% among the overall population, with better results against influenza B and influenza A (H1N1) viruses than influenza A(H3N2). Despite the difficulty in determining how effective the influenza vaccine may be, studies have shown that it is still a public health benefit; it was calculated that the flu vaccine prevented an estimated 7 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 deaths associated with influenza during the 2017–2018 season.23,24
Vaccination Timing
Recently, concerns have arisen regarding the need for annual influenza vaccinations and the optimal timing of influenza vaccination and vaccine effectiveness. Protective immunity generated by the vaccine typically takes up to 2 weeks to develop, with moderate effectiveness lasting about 6 months. Annual vaccines are designed to introduce the immune system to any mutations the virus has made. Repeated vaccinations were found to be effective in preventing severe and fatal influenza, especially in older adults, highlighting the importance of annual influenza vaccination.25
With the arrival of flu shots as early as July, the question is, how early is too early? It has been recommended to receive the influenza vaccine before the influenza season begins, typically by the end of October. However, many people are beginning to receive them as early as August due to the widespread availability. It is estimated that the influenza vaccine loses between 6% and 11% of its absolute effectiveness per month, depending on the influenza strain.26 Fears that patients may be receiving vaccines too early have led to studies evaluating the use of a compressed vaccine schedule by limiting the distribution of vaccines until the beginning of October. Using the 2014–2015 influenza season strain distributions, delaying vaccination to October or later resulted in a decrease of 11,423 cases, with projections of 481 fewer hospitalizations and 134 fewer deaths for people aged 65 years and older in the U.S. However, this statement assumes that the total number of vaccinations received by patients will remain the same despite the new restrictions on distribution and that the influenza peak will occur in February. Although the effects of this limited schedule on the total number of patients getting vaccinated are uncertain, a decrease of at least 3.5% in the 2014−2015 influenza season would reverse the benefits and result in more influenza cases.27
Secondly, if there were an early influenza peak, then a late vaccine schedule would result in more cases as well. Similar results were shown when flu vaccines were given early in the season during a late influenza peak.28 Recommending an optimal time for influenza vaccination is difficult; influenza seasons are unpredictable, and limiting distribution may result in fewer people receiving the vaccine. However, the CDC does recognize that getting vaccinated too early, such as in July or August, may be associated with reduced protection later in the influenza season, especially in older adults. The CDC recommends that individuals receive their vaccinations early in the fall, before the flu season begins, but no later than the end of October. Vaccinations beyond this date may still be beneficial, and vaccines should be offered throughout the entire influenza season.29
Adverse Effects
There are many misunderstandings about the influenza vaccine and its side effects. A common misconception about influenza vaccine is that it can cause influenza. Both the IIV and LAIV are safe and cannot cause the flu. The most common side effect of the IIV is soreness or redness at the site of injection. Systemic effects, including fever, malaise, and myalgias, may occur in a small percentage of individuals, most often in those who have had no previous exposure to the influenza virus antigens in the vaccine. These adverse reactions are usually mild and self-limiting, most resolving in 2 days or less. In clinical trials, HD-IIV injection site and systemic reactions were more frequent. Similarly, the aIIV is associated with more pain and tenderness at the injection site within the first 7 days. Studies have shown a small association between the influenza vaccine and Guillain-Barré syndrome; although rare, those with a history of this disorder should be cautious when weighing the benefits versus the risks.30-33
Every ingredient found in the vaccine has a purpose, whether it is providing immunity, preserving the product, or a necessary component of production. One ingredient found in multidose vials is the preservative thimerosal, which is metabolized to ethyl mercury. Following administration, ethyl mercury is found in much lower concentrations in the brain and has a much shorter elimination half-life compared with its commonly mistaken counterpart, methyl mercury. The small amounts used to preserve the vaccine do not cause mercury-associated adverse events, such as tremors. Formaldehyde is used to inactivate the viruses and is diluted during the later stages of the manufacturing process, but trace amounts may still be found in the vaccine. The concentration found in vaccines is much smaller than the amount appearing naturally in the body and, thus, is not a concern. Egg protein is used to allow the virus to grow and replicate before it is harvested. The ACIP provides recommendations regarding persons who are allergic to egg products (Table 2).34-37
Role of the Pharmacist
Pharmacists play an important role in combating the influenza virus. With a growing number able to vaccinate, pharmacists can not only help immunize but also educate the community and dispel misconceptions surrounding vaccines. With the flu season fast approaching, it is imperative that as many individuals as possible are vaccinated in a timely and safe manner.
REFERENCES
1. CDC. Products. Data briefs. Number 328; November 2018. Published November 29, 2018. www.cdc.gov/nchs/products/databriefs/db328.htm. Accessed February 6, 2019.
2. CDC. Estimated influenza Illnesses, medical visits, hospitalizations, and deaths in the United States; 2017–2018 influenza season. Published March 26, 2019. www.cdc.gov/flu/about/burden/2017-2018.htm. Accessed October 9, 2019.
3. CDC. What you should know for the 2017–2018 influenza season. www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed October 9, 2019.
4. Richards KA, Chaves FA, Alam S, Sant AJ. Trivalent inactivated influenza vaccines induce broad immunological reactivity to both internal virion components and influenza surface proteins. Vaccine. 2012;31(1):219-225.
5. Soema PC, Kompier R, Amorij JP, Kersten GF. Current and next generation influenza vaccines: formulation and production strategies. Eur J Pharm Biopharm. 2015;94:251-263.
6. Sridhar S, Brokstad KA, Cox RJ. Influenza vaccination strategies: comparing inactivated and live attenuated influenza vaccines. Vaccines. 2015;3(2):373-389.
7. Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev. 2013;26(3):476-492.
8. CDC. How influenza (flu) vaccines are made. Published July 10, 2019. www.cdc.gov/flu/prevent/how-fluvaccine-made.htm. Accessed October 10, 2019.
9. Hegde NR. Cell culture-based influenza vaccines: a necessary and indispensable investment for the future. Hum Vaccine Immunother. 2015;11(5):1223-1234.
10. CDC. Cell-based flu vaccines. Published October 11, 2019. www.cdc.gov/flu/prevent/cell-based.htm. Accessed October 15, 2019.
11. Cox MM, Hollister JR. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals. 2009;37(3):182-189.
12. FDA. Questions and answers - FluMist Quadrivalent (influenza virus vaccine live, intranasal). April 2019. www.fda.gov/vaccines-blood-biologics/vaccines/questions-and-answers-flumist-quadrivalent-influenza-virus-vaccine-live-intranasal. Accessed October 15, 2019.
13. CDC. Selecting viruses for the seasonal flu vaccine. Published September 4, 2018. www.cdc.gov/flu/prevent/vaccine-selection.htm. Accessed October 15, 2019.
14. CDC. Fluzone high-dose seasonal influenza vaccine. Published September 6, 2019. www.cdc.gov/flu/prevent/qa_fluzone.htm. Accessed October 15, 2019.
15. Lee JKH, Lam GKL, Shin T, et al. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Rev Vaccines. 2018;17(5):435-443.
16. DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635-645.
17. Durando P, Icardi G, Ansaldi F. MF59-adjuvanted vaccine: a safe and useful tool to enhance and broaden protection against seasonal influenza viruses in subjects at risk. Expert Opin Biol Ther. 2010;10(4):639-651.
18. Tsai TF. Fluad®-MF59®-adjuvanted influenza vaccine in older adults. Infect Chemother. 2013;45(2):159-174.
19. Domnich A, Arata L, Amicizia D, et al. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: a systematic review and meta-analysis. Vaccine. 2017;35(4):513-520.
20. ClinicalTrials.gov. FLUAD vs. Fluzone high-dose study. https://clinicaltrials.gov/ct2/show/NCT03183908. Accessed October 16, 2018.
21. Grohskopf LA, Alyanak, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices — United States, 2019–20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
22. Kim DK, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2019. MMWR Morb Mortal Wkly Rep. 2019;68:115-118.
23. CDC. Vaccine effectiveness. How well does the flu vaccine work? Seasonal influenza (Flu). Published October 12, 2018. www.cdc.gov/flu/about/qa/vaccineeffect.htm. Accessed October 16, 2018.
24. Rolfes MA, Flannery B, Chung J, et al. Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis. February 2, 2019; Epub ahead of print.
25. Casado I, Domínguez Á, Toledo D, et al. Repeated influenza vaccination for preventing severe and fatal influenza infection in older adults: a multicentre case-control study. CMAJ. 2018;190(1):E3-E12.
26. Ferdinands JM, Fry AM, Reynolds S, et al. Intraseason waning of influenza vaccine protection: evidence from the US Influenza Vaccine Effectiveness Network, 2011–2012 through 2014–2015. Clin Infect Dis. 2017;64(5):544-550.
27. Smith KJ, France G, Nowalk MP, et al. Compressed influenza vaccination in U.S. older adults: a decision analysis. Am J Prev Med. 2019;56(4):e135-e141.
28. Ferdinands JM, Alyanak E, Reed C, Fry AM. Waning of influenza vaccine protection: exploring the trade-offs of changes in vaccination timing among older adults. Clin Infect Dis. June 29, 2019. Epub ahead of print.
29. CDC. Everyone 6 months of age and older should get a flu vaccine. Published October 11, 2019. www.cdc.gov/flu/prevent/vaccinations.htm. Accessed October 15, 2019.
30. CDC. Seasonal influenza vaccine safety: a summary for clinicians. Published September 18, 2019. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed October 15, 2019.
31. CDC. Safety of influenza vaccines. Published September 12, 2019. www.cdc.gov/flu/professionals/acip/safety-vaccines.htm. Accessed October 15, 2019.
32. Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis. 2013;13(9):769-776.
33. Polakowski LL, Sandhu SK, Martin DB, et al. Chart-confirmed Guillain-Barré syndrome after 2009 H1N1 influenza vaccination among the Medicare population, 2009–2010. Am J Epidemiol. 2013;178(6):962-973.
34. CDC. Vaccines: Vac-Gen/additives in vaccines fact sheet. Published August 7, 2019. www.cdc.gov/vaccines/vac-gen/additives.htm. Accessed October 15, 2019.
35. CDC. Thimerosal in vaccines. Thimerosal concerns. Vaccine safety. Published January 24, 2019. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed October 15, 2019.
36. FDA. Common ingredients in U.S. licensed vaccines. April 2019. www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines. Accessed October 15, 2019.
37. CDC. Flu vaccine and people with egg allergies. Published December 28, 2017. www.cdc.gov/flu/prevent/egg-allergies.htm. Accessed October 15, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.
How can flu be prevented?
Getting vaccinated annually is the best way to prevent contracting and spreading the flu. Other options include washing hands regularly, minimizing contact with those who are already infected, and avoiding touching the face.
How do vaccines work?
Seasonal flu vaccines introduce a weakened or inactivated (dead) form of influenza that causes our bodies to develop antibodies that will provide protection against future exposures of certain strains of the virus in the vaccine.
When should I receive my vaccine?
The CDC recommends getting your vaccine before the flu season begins, generally before the end of October.
Where can I get vaccinated?
Contact your local pharmacy or primary healthcare provider; many pharmacies, doctor's offices, or health clinics offer vaccinations.
Why do I need a vaccine every year?
The immunity conferred by the flu vaccine declines over time. The influenza virus is also constantly changing, so it is important to get an annual vaccine to “update” your immune system.
Can the flu shot give me the flu?
Neither the killed nor weakened form are contagious, meaning they will not give you the flu.
What are some side effects?
Possible side effects may include soreness or redness at the site of injection as well as low-grade fever and body aches.
Do flu shots contain mercury?
Thimerosal is a mercury-based preservative found in some versions of the flu vaccine. However, thimerosal is turned into ethyl mercury that is quickly removed from the body, as opposed to the harmful methyl mercury that is typically associated with mercury poisoning.
What are some of the benefits?
The influenza vaccine will lower your chances of contracting the flu and prevent hospitalizations associated with the flu. It has also been shown to lower the risk of heart-related events due to contracting the flu in those with certain cardiovascular diseases. Patients with diabetes and chronic lung disease have been shown to have decreased hospitalizations as well. Lastly, newborns may benefit if the mother receives a vaccine while pregnant because antibodies are passed to the developing baby during pregnancy.