US Pharm. 2019;44(8):24-28.
ABSTRACT: Bacterial prostatitis is a complex genitourinary infection that is characterized by whether it is acute or chronic in nature. The sequestered prostate gland hinders the penetration of many antibiotics, making this condition notoriously difficult to treat. Fluoroquinolones have been the cornerstone of treatment, although poor tolerability, adherence to prolonged treatments, and widespread emergence of resistance pose an urgent threat to their use for this condition. Community pharmacists play a crucial role in the care of patients with prostatitis. Through advocating for antimicrobial stewardship, community pharmacists can collaborate with physicians to optimize antibiotic therapy, mitigate adverse effects, and counsel patients on their treatment and supportive-care measures.
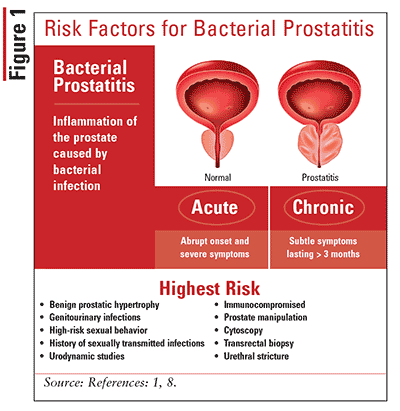
Bacterial prostatitis is a genitourinary infection characterized by inflammation and swelling of the prostate gland that can be either acute or chronic in nature.1 Acute bacterial prostatitis (ABP) is distinguished by an abrupt onset of local (pelvic pain, dysuria) and systemic (fevers, chills, malaise) symptoms accompanied by a urinary tract infection (UTI). Chronic bacterial prostatitis (CBP) results from relapsing or recurrent UTIs with the persistence of the same bacterial strain.2,3 CBP is a subtle illness, and patients’ symptoms wax and wane over time, making the identification challenging for clinicians. Given that its symptomatology mimics those of other common genitourinary conditions (i.e., UTIs, benign prostatic hypertrophy, bladder obstruction), patients are frequently misdiagnosed and the initiation of treatment is delayed.
If left untreated, the consequences of bacterial prostatitis can be severe and range from urosepsis in ABP to erectile dysfunction, infertility, and chronic pelvic pain in CBP.1,4 ABP responds favorably to antimicrobial treatment; however, effective treatment of CBP is hampered by the limited availability of antimicrobials that penetrate noninflamed prostatic tissue. Men living with prostatitis can have impairment in both physical and mental domains of their health-related quality of life, which underscores the importance of appropriate recognition and treatment to improve patient outcomes.
Etiology
Bacteria can gain entry to the prostate by ascending through the urethra after infected urine is refluxed into the prostatic ducts.3 Alternatively, infection can result from hematogenous seeding or direct bacterial inoculation during surgical procedures.4 In a majority of cases, bacterial prostatitis occurs secondary to acute or recurrent UTIs, and less commonly, from epididymitis, urethritis, or infected prostatic calculi (Figure 1).1 Men with chronic indwelling catheters, with uncontrolled diabetes, or who are immunosuppressed may be predisposed to developing prostatitis because the presence of foreign devices, hyperglycemia, and impaired immune responses favor the exponential growth of micro-organisms.4 Escherichia coli is the predominant pathogen isolated, but an increasing role of the gram-positive organism Enterococcus has been reported.5 E coli strains responsible for prostatitis exhibit distinct virulence factors that promote the formation of resistant biofilms, which have been identified as main drivers for persistent infection leading to CBP.3 It is important to recognize that the emergence, spread, and persistence of multidrug-resistant organisms, most notably among uropathogens such as E coli, has drastically shifted the epidemiology of genitourinary infections and can play a critical role in the microbiology of prostatitis. Sexually transmitted infections, including gonorrhea, chlamydia, and Ureaplasma urealyticum, are also implicated in prostatitis, particularly in young, sexually active men and those living with HIV. Fungi, mycobacteria, and viruses are infrequent pathogens but may be isolated in the immunocompromised host.4
Diagnosis
The essential steps to confirm the diagnosis of prostatitis begin with a thorough physical examination and medical history, followed by microbiological cultures to isolate the causative organism. Although community pharmacists are not intimately involved in the diagnostic evaluation, it is imperative to understand the features that distinguish ABP from CBP, as well as from a UTI, because both treatment and treatment duration drastically differs among these conditions. The presence of focal symptoms such as pelvic or perineal pain is suggestive of prostatic involvement and is an uncommon finding in patients with cystitis.4
ABP is characterized by an abrupt onset of urinary urgency, frequency, incomplete voiding, and pelvic pain. The presence of systemic symptoms (fever, malaise, chills) should prompt the community pharmacist to triage patients to seek urgent medical care for empiric antibiotics, as this condition can rapidly pro-gress to urosepsis. A gentle digital rectal exam is often used to examine inflammation of the prostate and can elicit exquisite pain and tenderness.3,4 A midstream urine specimen is performed to isolate the causative organism, with culture and sensitivity tests sent to direct antibiotic de-escalation. A prostatic massage to obtain expressed prostatic secretions (EPS) should be avoided due to the risk of causing bacterial translocation and subsequent bacteremia.1
The diagnosis of CBP is considerably more difficult because the symptoms are less acute, resemble other syndromes, and can arise and vanish unpredictably. A history of recurrent UTIs and associated genitourinary symptoms that persist for 3 months or longer is commonly associated with CBP.3 Urine cultures are important but may be negative because bacteriuria is not always present. Unlike ABP, bacterial localization tests through the collection of EPS are fundamental in diagnosing CBP.1,4 A 2-glass test is performed by obtaining pre- and post-prostatic massage urine specimens to compare the presence of leukocytes and bacterial growth. The diagnosis is confirmed based on a 10-fold increase in bacteria in the post-prostatic urine and the detection of leukocytes. In cases of recurrence, imaging can be important to identify prostatic calcification or abscess, as their presence can serve as a reservoir for infection.4
Treatment Overview
A focus of care in the treatment of bacterial prostatitis aims to eradicate the infecting organism to eliminate the risk of treatment relapse. The acuity of ABP requires urgent initiation of oral or IV antimicrobials, with de-escalation to target the isolated uropathogen once culture and sensitivity results are available. The optimal duration of treatment for ABP and CBP is not well studied. In ABP, most patients receive 2 to 4 weeks of antimicrobial therapy, with patients who present with severe illness, who have concomitant bacteremia, or who are treated with suboptimal antibiotics receiving longer durations.2,3
CBP is a subacute illness where delaying initiation of antibiotics until culture and sensitivity results are available is prudent, so that the causative organism is not eradicated before it can be identified.3 Eradication rates in CBP are low, and treatment for up to 4 to 6 weeks is recommended. Recurrence rates in CBP are as high as 25% to 50%, and close follow-up is necessary.4 Treatment with low-dose antimicrobials for 6 months has been suggested to reduce the risk of recurrences; however, this practice should be carefully weighed against the risk of adverse effects of long-term antimicrobial therapy and inducing antibiotic resistance.5,6 Compared with antimicrobial monotherapy, adjunctive treatment with alpha-blockers has been demonstrated to reduce the risk of recurrences and ameliorate the obstructive pain symptoms associated with bacterial prostatitis.1
Antimicrobial Therapy
Achieving antibiotic concentrations sufficient to inhibit bacterial growth within the prostate is a critical factor that drives the efficacy of treatment. Drug delivery is markedly hindered since the prostate is a sequestered site surrounded by a lipophilic epithelium and nonporous capillaries and lacks active transport mechanisms.6 Favorable pharmacokinetic properties that govern the penetration of a selected antimicrobial include low protein binding, low degree of ionization, low molecular weight, and high lipid solubility.1,6 Unfortunately, only a handful of antimicrobials exhibit these characteristics, including fluoroquinolones, trimethoprim, and to a lesser extent, macrolides, clindamycin, and tetracyclines. An exception is during the initial phase of ABP, because the acutely inflamed prostate allows the penetration of antimicrobials with otherwise unfavorable pharmacokinetics, such as many beta-lactams and aminoglycosides. Nevertheless, as the inflammation subsides, the noninflamed prostate renders most antibiotics ineffective.1,2
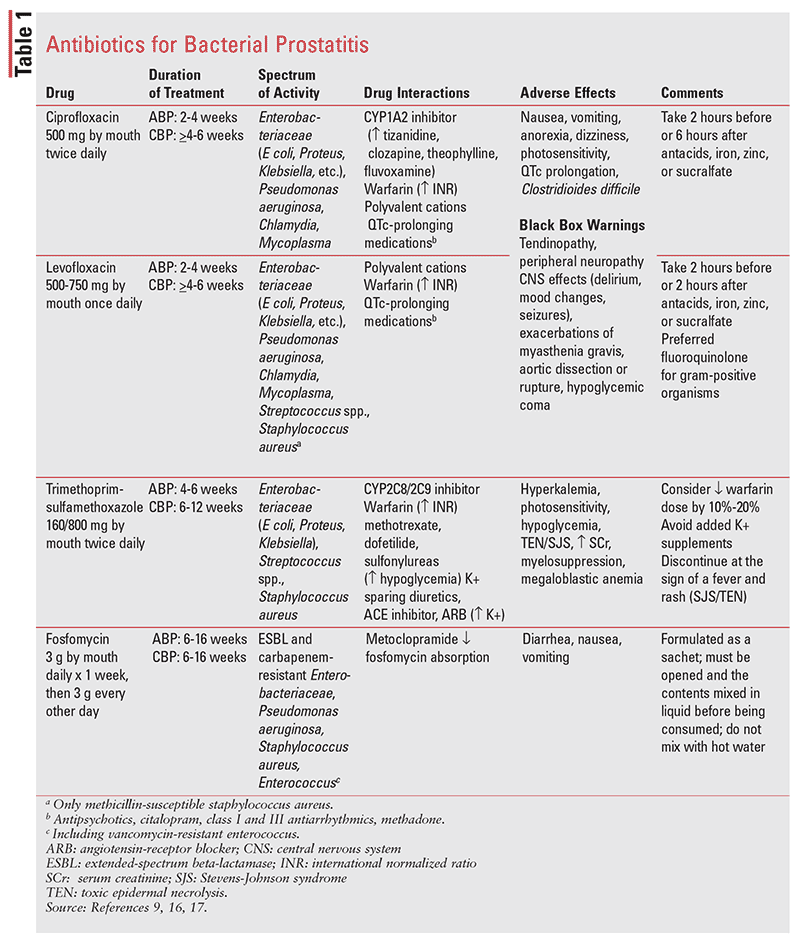
Based on their pharmacokinetic properties and high oral bioavailability, fluoroquinolones are the most commonly prescribed agents for bacterial prostatitis. Fluoroquinolones exist in a zwitterionic form that facilitates passive diffusion into the prostate, achieving concentrations 2- to 3-fold higher than in the plasma.4,6 Ciprofloxacin and levofloxacin have the most evidence for bacterial prostatitis treatment to date and demonstrate comparable efficacy across clinical studies.7,8 Overall, cure rates for E coli and other Enterobacteriaceae infections approximate 60% to 80% after a minimum 4-week course of fluoroquinolone therapy.2 Prostatitis due to Pseudomonas aeruginosa and enterococci shows lower response to fluoroquinolone therapy. Of note, a majority of this data was collected prior to the recognition of the diffuse resistance to fluoroquinolones and should be viewed in a contemporary perspective, which necessitates culture collection with susceptibility verification.
The use of trimethoprim-sulfamethoxazole (TMP-SMX) for empiric treatment as an alternative to fluoroquinolones has been suggested in the setting of intolerance or when local fluoroquinolone resistance exceeds 10%. Of note, cure rates are suboptimal at 30% to 50%; this has been proposed to be due in part to the inability of sulfamethoxazole to penetrate prostatic tissue, reducing its synergetic activity with trimethoprim.6,8 Therefore, trimethoprim monotherapy can be used, particularly in patients with sulfa allergies. Lower cure rates warrant treatment extension to 12 weeks for CBP.1,3 Local resistance of 20% or more is the recommended threshold to avoid TMP-SMX for empiric therapy. See Table 1 for dosing, spectrum of activity, and adverse effects for antimicrobial treatments.
Adverse Effects
Over the past decade, the FDA has issued multiple new warnings regarding the potentially disabling adverse effects of fluoroquinolones. Within the past 5 years alone, the FDA added and strengthened warnings regarding the risk of aortic dissection, aortic rupture, severe hypoglycemia, psychiatric effects, and tendinopathy.9-11 There is a wide spectrum of central nervous system manifestations ranging from nervousness, agitation, and insomnia to seizures and delirium.9 Older adults are particularly susceptible and should be under close supervision as these symptoms may be mistakenly overlooked for other geriatric syndromes. Cardiotoxicity manifesting as QTc prolongation can occur, and pharmacists should assess for patients who have low potassium and magnesium, heart failure, arrhythmias, and QTc-prolonging medications to minimize the risk of developing torsades de pointes.10 Adverse effects are relatively common, and fluoroquinolones are important causes of Clostridioides difficile infections owing to their high potential for collateral damage.
There have been rising numbers of alerts concerning the potential for severe glucose alterations, with an emphasis on the potential for hypoglycemia and hypoglycemic comas. Exercise caution when using fluoroquinolones in patients with risk factors for severe hypoglycemia, such as older adults, patients with diabetes on antihyperglycemics, or those with chronic kidney disease.10 Fluoroquinolone-induced peripheral neuropathy is a disabling, irreversible toxicity that can occur at any point during treatment and may persist long after discontinuation.9 Tendinopathy is a well-recognized adverse effect that may occur as early as the first few days of therapy and affects the Achilles tendon in the majority of cases. The association is greatest in patients receiving corticosteroids, prior transplant recipients, and patients older than age 60 years, although fluoroquinolone use in young adults should also raise concern because vigorous exercise can potentiate tendon rupture. Lastly, fluoroquinolones can predispose patients to phototoxicity; skin hygiene and liberal use of sunscreen should be advised.9,10
TMP-SMX is generally well-tolerated, with the most common adverse effects including gastrointestinal intolerance and mild skin reactions.12 Stevens-Johnson syndrome and toxic epidermal necrolysis are rare but life-threatening skin reactions that warrant immediate drug cessation and referral to a hospital. Myelosuppression is both dose- and duration-dependent, and it is prudent to monitor for it in patients taking TMP-SMX for prostatitis, given the prolonged durations used.13 TMP inhibits the tubular secretion of creatinine, resulting in a transient elevation in serum creatinine by ~10% without a true reduction in the glomerular filtration rate.12,13 The structural similarity of trimethoprim to amiloride leads to inhibition of potassium elimination in the nephron, resulting in hyperkalemia in some patients. When used in combination with angiotensin receptor blockers, ACE inhibitors, or potassium-sparing diuretics, the consequences may be fatal, with reports suggesting this combination increased the risk of hospitalization for hyperkalemia by sevenfold, making the periodic monitoring of electrolytes a requirement.13
Other Therapeutic Options
Although fluoroquinolones have remained the antibiotic workhorses for prostatitis, increased resistance to these agents currently limits their widespread utility.5 Fluoroquinolone resistance rates to Enterobacteriaceae have escalated in recent years to more than 30% nationally and eclipsed TMP-SMX resistance in some areas.14 Resistant prostatic infections are notoriously difficult to treat given the sequestration of the prostate gland, underscoring the necessity of new treatment options.
Although its FDA-labeled indication is limited to the treatment of acute uncomplicated cystitis, which by definition excludes men, fosfomycin has been evaluated off-label for the treatment of CBP caused by multidrug-resistant organisms (MDRO). Fosfomycin has several advantages over conventional therapies owing to its low risk of collateral damage and in vitro activity against a wide array of resistant uropathogens including extended-spectrum beta-lactamases, carbapenem-resistant Enterobacteriaceae, and Enterococcus faecalis. In TMP-SMX or ciprofloxacin-resistant E coli, fosfomycin maintains impressive activity at 99.7% and 96.1%, respectively.5 In addition, the small molecular size, low protein binding, and high lipophilicity allows for therapeutic concentrations well above those needed to overcome the minimum inhibitory concentrations of MDRO.10 A prospective observational study evaluating a 6- to 12-week course of fosfomycin for MDRO demonstrated clinical and microbiological cure rates of 82% and 86%, respectively.15 It is essential to acknowledge that the efficacy of fosfomycin is contingent upon dose optimization. The highest cure rates have been achieved with the use of 3 grams daily for 1 week, and then de-escalation to every 48 hours for a total of 6 to 16 weeks of treatment.5,15 Extended-interval therapy every 72 hours, or single doses of more than 3 g more frequently than every 48 hours, resulted in a higher frequency of clinical failure or adverse effects, respectively. Data thus far are encouraging, though there are some practical limitations to the widespread use of fosfomycin. The average wholesale price of fosfomycin is ~$100 per dose, which is substantially higher than other oral antibiotics. The economic burden can be significant, especially considering the extended duration of treatment and limited insurance coverage.16 The poor bioavailability of fosfomycin limits its use in patients with ABP if there is a concern for bacteremia or pyelonephritis.
In cases of severe bacterial prostatitis, IV antibiotics may be required. Ceftriaxone or ertapenem are attractive options, as these agents achieve high prostate concentrations and can be administered once daily, making them convenient options for outpatient therapy.5 Regarding alternative beta-lactams, their ability to concentrate in the prostate varies among agents, so knowledge of each agent’s pharmacokinetics is required before one is selected. Aminoglycosides have excellent activity against the causative pathogens for prostatitis but penetrate poorly into prostatic tissue.6
Role of the Community Pharmacist
Community pharmacists can serve as advocates to improve the quality of care for patients with bacterial prostatitis. One common role for pharmacists is in triage. Pharmacists hearing complaints that resemble those of bacterial prostatitis should refer patients to acute-care settings. For example, a conversation with a patient with benign prostatic hyperplasia who is refilling a medication may reveal a change in symptomatology that could indicate that infection of the prostate has occurred.
Prolonged treatment durations and the high frequency of relapses can be burdensome to patients, which can have a significant impact on their quality of life. Despite the alarming rise of resistance and adverse effects, fluoroquinolones remain the foundation of treatment for prostatitis for susceptible infections. Given the attention that fluoroquinolone adverse effects have received, patients may be reluctant to take them for prolonged courses, leading to compromised adherence. Adherence is a crucial antimicrobial stewardship measure to prevent the dissemination of drug resistance. Pharmacists are positioned to have an informed discussion to address patient concerns, counsel about warning signs with the emergence of adverse effects, and properly educate about medication risks and benefits.
REFERENCES
1. Khan FU, Ihsan AU, Khan HU, et al. Comprehensive overview of prostatitis. Biomed Pharmacother. 2017;94:1064-1076.
2. Wagenlehner FM, Pilatz A, Bschleipfer T, et al. Bacterial prostatitis. World J Urol. 2013;31(4):711-716.
3. Lipsky BA, Byren I, Hoey CT. Treatment of bacterial prostatitis. Clin Infect Dis. 2010;50(12):1641-1652.
4. Gill BC, Shoskes DA. Bacterial prostatitis. Curr Opin Infect Dis. 2016;29(1):86-91.
5. Zhanel GG, Zhanel MA, Karlowsky JA. Oral fosfomycin for the treatment of acute and chronic bacterial prostatitis caused by multidrug-resistant Escherichia coli. Can J Infect Dis Med Microbiol. 2018:1404813.
6. Charalabopoulos K, Karachalios G, Baltogiannis D, et al. Penetration of antimicrobial agents into the prostate. Chemotherapy. 2003;49(6):269-279.
7. Perletti G, Marras E, Wagenlehner FM, Magri V. Antimicrobial therapy for chronic bacterial prostatitis. Cochrane Database Syst Rev. 2013;(8):CD009071.
8. Nickel JC. Inflammatory and pain conditions of the male genitourinary tract: prostatitis and related pain conditions, orchitis, and epididymitis. In: McDougal WS, Wein AJ, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier. 2016:Chapter 13.
9. Levofloxacin prescribing information. Sunrise, FL: Cipla USA, Inc; February 2019.
10. Roberts R. Adverse reactions to fluoroquinolones. Emerg Med News. 2008;30(10):16-18.
11. Lee CC, Lee MT, Hsieh R, et al. Oral fluoroquinolone and the risk of aortic dissection. J Am Coll Cardiol. 2018;72(12):1369-1378.
12. Sulfatrim DS sulfamethoxazole/trimethoprim product monograph. Vaughan, Ontario, Canada: AA Pharma Inc; January 2019.
13. Ho JM, Juurlink DN. Considerations when prescribing trimethoprim-sulfamethoxazole. CMAJ. 2011;183(16):1851-1858.
14. Spellberg B, Doi Y. The rise of fluoroquinolone-resistant Escherichia coli in the community: scarier than we thought. J Infect Dis. 2015;212(12):1853-1855.
15. Karaiskos I, Galani L, Sakka V, et al. Oral fosfomycin for the treatment of chronic bacterial prostatitis. J Antimicrob Chemother. February 22, 2019. {Epub ahead of print].
16. Monurol (fosfomycin) prescribing information. Madison, NJ: Allergan USA, Inc; May 2018.
17. Ciprofloxacin prescribing information. Mississauga, Ontario, Canada; February 2019.
To comment on this article, contact rdavidson@uspharmacist.com.