US Pharm. 2020;45(5)(Specialty&Oncology suppl):3-8.
ABSTRACT: In recent years, several new targeted therapy options have been developed to combat resistance to traditional endocrine therapy for advanced breast cancer. Three CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) have been approved for patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer. Each agent has demonstrated its efficacy, but differences between the three drugs exist, particularly in their adverse-event profiles.
Breast cancer is the most common type of cancer in women, with more than 276,000 new cases estimated in 2020.1 Hormone receptor–positive (HR+) breast cancer, which accounts for more than 70% of these cases, historically has been treated with endocrine therapy (ET), such as selective estrogen receptor inhibitors and aromatase inhibitors (AIs). Although ET remains the backbone of HR+ breast cancer treatment, resistance develops in up to 50% of patients with advanced breast cancer.2 To combat resistance, targeted therapies have been approved for HR+ breast cancer.3,4
Cyclin-dependent kinase (CDK) inhibitors, the newest class of interest for advanced breast cancer, work by specifically inhibiting CDK4/6 proteins and blocking the transition from the G1 to the S phase of the cell cycle. This drug class inhibits kinase activity, which phosphorylates the retinoblastoma protein pathway. By blocking this path, CDK4/6 inhibitors are able to block cell-cycle progression in the middle of the G1 phase and prevent cancer cell progression. In recent years, three CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) have been approved as combination therapies for HR+, human epidermal growth factor receptor 2–negative (HER2–), advanced or metastatic breast cancers.5-8 This article reviews these agents’ differences (TABLE 1), phase III clinical trials (TABLE 2), and adverse-event (AE) profiles (TABLE 3).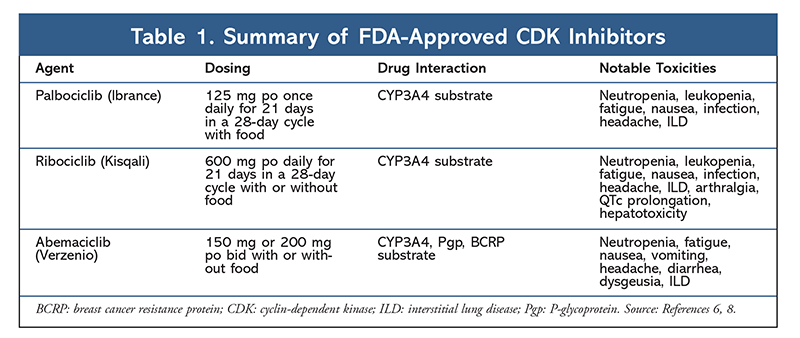
Palbociclib
In 2015, palbociclib became the first CDK4/6 inhibitor to receive FDA approval for HR+, HER2– breast cancer treatment (in combination with an AI as initial ET in postmenopausal women or in men, or in combination with fulvestrant in patients with disease progression following ET).6,9-14 Palbociclib initially received approval after positive results from the phase II study PALOMA-1, which compared palbociclib plus letrozole with letrozole alone as initial therapy for ET-naïve advanced breast cancer patients.9
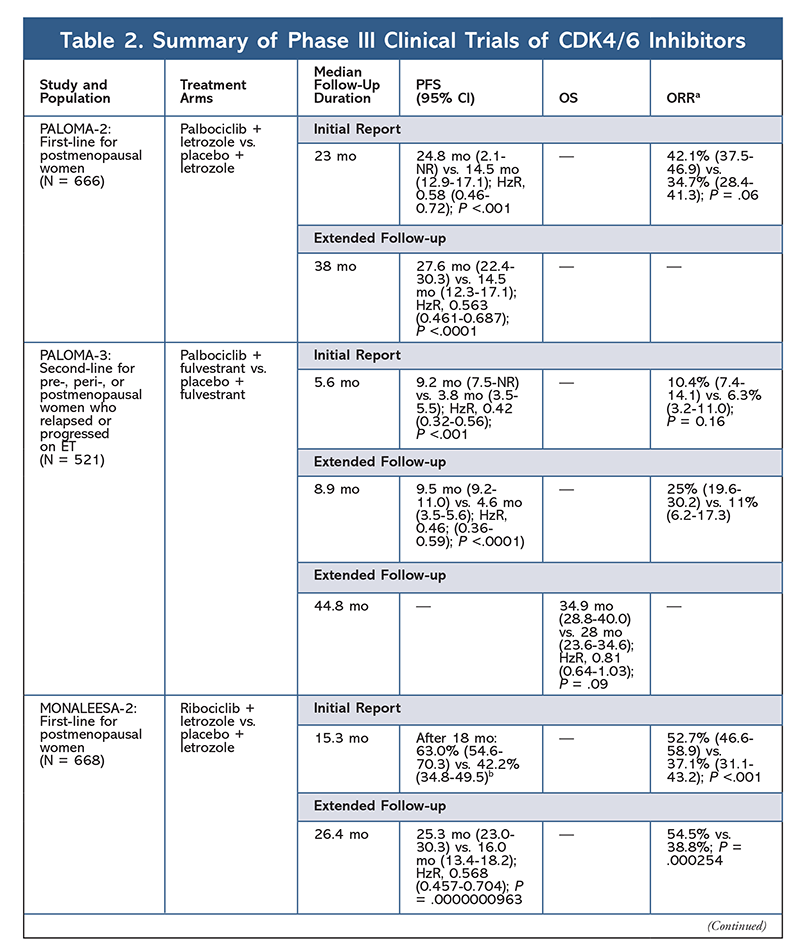
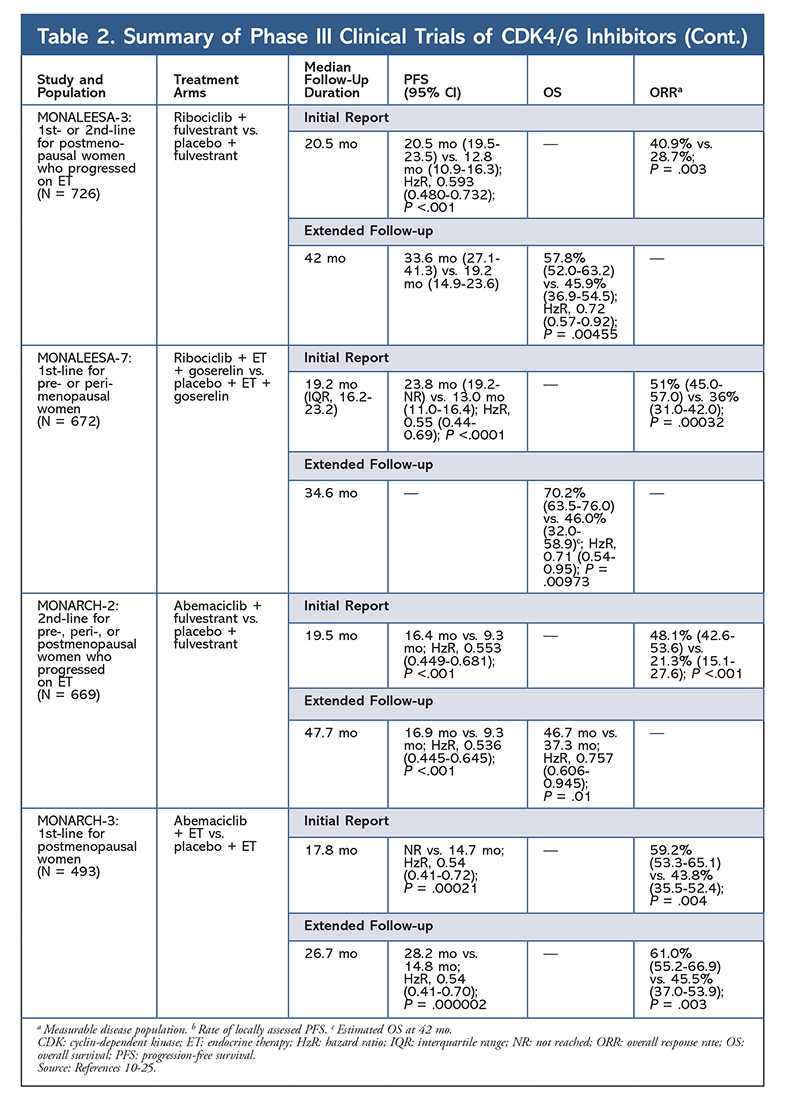
These results led to two phase III trials further evaluating palbociclib’s effect in this patient population. Initial results of PALOMA-2 confirmed the effects of palbociclib plus letrozole, demonstrating improved progression-free survival (PFS) versus letrozole alone (24.8 mo vs. 14.5 mo, respectively; hazard ratio [HzR] for disease progression or death, 0.58; 95% CI, 0.46-0.72; P <.001). A long-term follow-up study at 38 months further confirmed palbociclib’s effect on PFS, with an increase to 27.6 months versus 14.5 months for letrozole alone (HzR, 0.563; 95% CI, 0.461-0.687; P <.0001). The follow-up study also reported consistent PFS improvement with palbociclib plus letrozole across all studied subgroups, as well as delayed median time to initiation of subsequent therapy. However, overall survival (OS) data for the initial and follow-up reports were immature, and enrolled patients are being followed for survival.10,11
Shortly after the start of PALOMA-2, another phase III trial, PALOMA-3, was begun to assess the safety and efficacy of palbociclib plus fulvestrant in premenopausal or postmenopausal women with HR+ advanced breast cancer that progressed on prior ET. The initial report after 5.6 months of follow-up showed an improvement in median PFS (9.2 mo vs. 3.8 mo), with a 58% reduction in progression (HzR, 0.42; 95% CI, 0.32-0.56; P <.001).12 A prespecified analysis of OS in the PALOMA-3 trial, using a median follow-up of 44.8 months, found that the differences in OS for the entire intent-to-treat group did not reach statistical significance, but the combination did prolong OS by 10 months in patients who were sensitive to previous ET.14
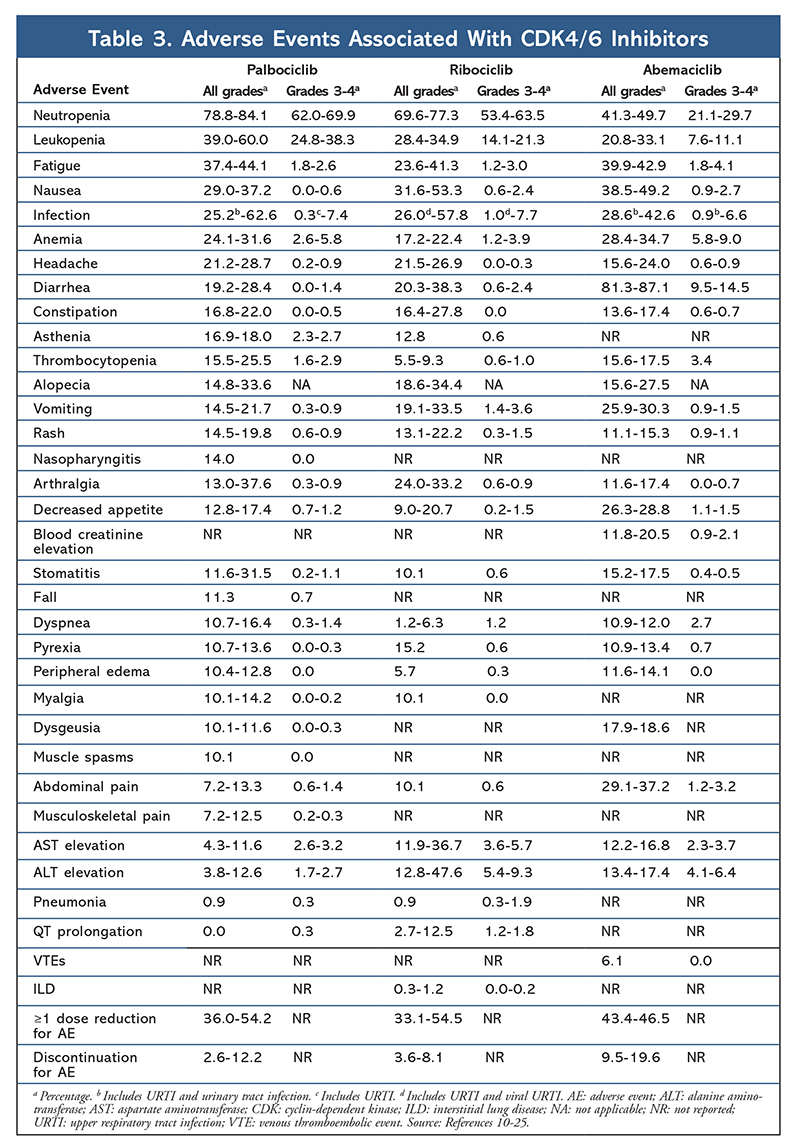
The AE profile remained consistent across all trial reports (TABLE 3), with all-grade neutropenia (78.8%-84.1%), leukopenia (39%-60%), and fatigue (37.4%-44.1%) being the most frequently reported AEs. Rates of neutropenia were high; however, febrile neutropenia was rare (up to 2%). The overall tolerability of palbociclib was considered to be manageable, and the agent resulted in positive quality-of-life data when used in combination therapy compared with endocrine monotherapy.9,14
Ribociclib
A second CDK4/6 inhibitor, ribociclib, was FDA approved in 2017 with a broader indicated patient population. Ribociclib is approved for postmenopausal and pre- or perimenopausal women with HR+, HER2– advanced or metastatic breast cancer (as combination therapy with an AI as initial ET or, in postmenopausal women, combined with fulvestrant as initial ET or following disease progression on ET).7
Ribociclib first received FDA approval after the MONALEESA-2 trial found that PFS was improved when the drug was given in combination with letrozole versus letrozole alone. Rates of locally assessed PFS versus letrozole alone after 18 months were 63.0% (95% CI, 54.6-70.3) and 42.2% (95% CI, 34.8-49.5), respectively.15 An extended follow-up report at 26.4 months further demonstrated the efficacy and tolerability of the combination.16 Despite the improvement in PFS, OS data for MONALEESA-2 remain immature.
Ribociclib is also being investigated (in MONALEESA-3) in combination with fulvestrant in treatment-naïve patients and those who relapsed after ET. The initial report revealed improved PFS for the combination compared with fulvestrant alone (20.5 mo vs. 12.8 mo, respectively; HzR, 0.593; 95% CI, 0.480-0.732; P <.001), which resulted in FDA approval of this combination.17 A second, and the most recent, CDK4/6 inhibitor report, which had 21.6 additional months of follow-up, further demonstrated the consistent PFS improvement. This report also proved the OS benefit of ribociclib plus fulvestrant compared with fulvestrant alone, with an estimated OS at 42 months of 57.8% versus 45.9%, respectively, and a 28% reduction in relative risk of death (HzR, 0.72; 95% CI, 0.57-0.92; P = .00455).18
A third clinical trial, MONALEESA-7, is currently the only phase III trial to evaluate the efficacy and safety of a CDK4/6 inhibitor as first-line therapy for pre- or perimenopausal women who have metastatic breast cancer. In this trial, ribociclib plus tamoxifen or a nonsteroidal AI (NSAI)—such as letrozole or anastrozole—plus goserelin significantly improved PFS by 23.8 months versus 13 months for the placebo group (HzR, 0.55; 95% CI, 0.44-0.69; P <.0001), and the combination led to the expanded indication that includes pre- and perimenopausal women.19 An extended follow-up report demonstrated the OS benefit of combination ribociclib plus tamoxifen or an NSAI plus goserelin compared with placebo, with an estimated 42-month OS of 70.2% versus 46.0%.20
The toxicity profile for ribociclib is highly similar to that of palbociclib, with neutropenia and leukopenia being the highest all-grade and severe (grades 3/4) AEs reported, although at lower rates than for palbociclib (TABLE 3).15-20 Febrile neutropenia was rare, and no new safety concerns arose with longer exposure to ribociclib in any of the extended follow-up reports.
Abemaciclib
The third CDK4/6 inhibitor to be FDA approved is abemaciclib. This agent is indicated in combination with an AI as initial ET in postmenopausal women with advanced breast cancer, or in combination with fulvestrant in pre-, peri-, or postmenopausal women with disease progression following ET. Abemaciclib is also approved as monotherapy in men and women with disease progression following ET and prior chemotherapy in the metastatic setting.6 The agent initially gained approval based on results from MONARCH-1 demonstrating promising clinical activity after 12 months, with an overall response rate (ORR) of 19.7% (95% CI, 13.3-27.5) and a median PFS of 6 months.5,21 During this time, abemaciclib was also being investigated in MONARCH-2 for pre-, peri-, and postmenopausal women who progressed on ET. This study demonstrated statistically significant PFS benefit with a combination of abemaciclib plus fulvestrant versus fulvestrant alone (16.4 mo vs. 9.3 mo; HzR, 0.553; 95% CI, 0.449-0.681; P <.001).22 An extended follow-up study with a median duration of 47.7 months reported slightly improved PFS results and OS benefit with the abemaciclib combination versus placebo versus fulvestrant (46.7 mo vs. 37.3 mo; 95% CI, 0.606-0.945; P = .01).23
Shortly after initiation of the two abemaciclib trials, MONARCH-3 was started. This trial found a statistically significant improvement in PFS and ORR with abemaciclib plus an NSAI compared with the placebo-plus-NSAI arm as first-line treatment for postmenopausal women. An updated report with 8.9 months of additional follow-up further confirmed the PFS and ORR results of the initial analysis; however, the OS data remain immature, and final OS analysis will be conducted after 315 events.24,25
Overall, abemaciclib has been noted to be the most potent CDK4/6 inhibitor, with excellent central nervous system activity due to its structure, allowing it to cross the blood-brain barrier and remain on target longer.5 This may be beneficial for patients with brain metastases. Although abemaciclib also has high percentages of hematologic AEs, these rates are much lower than those for other CDK4/6 inhibitors. Abemaciclib is associated with more gastrointestinal toxicities, such as diarrhea, than other CDK4/6 inhibitors (all grades: 81.3%-87.1%; grades 3-4: 9.5%-14.5%).22-25
The Pharmacist’s Role
Overall, the AE profiles of the various CDK 4/6 inhibitors are similar, but each drug has unique AEs. The most common AEs reported with CDK4/6 inhibitors are neutropenia, leukopenia, fatigue, nausea, infection, arthralgia, anemia, headache, and diarrhea (TABLE 3). Aside from neutropenia and leukopenia, the majority of patients reported grade 1 or 2 AEs. Grade 3 or 4 neutropenia and leukopenia, which were very common with all CDK inhibitors, particularly palbociclib and ribociclib, were managed mostly with dose interruption and/or reduction. The patient’s CBC should be monitored at baseline, every 2 weeks for the first two cycles, at the beginning of each subsequent four cycles, and as clinically necessary for bone marrow suppression.6-8 Falls, muscle spasms, and nasopharyngitis are associated with palbociclib, but are mild in most cases.10,11,14 A low incidence of QT prolongation has been reported with ribociclib.15-20 Abemaciclib was associated with a significantly higher incidence of diarrhea compared with palbociclib and ribociclib; cases occurred mostly during the first cycle and could be managed with antidiarrheal therapy.22-25 Abemaciclib was associated with serum creatinine elevation and venous thromboembolic events (mostly mild), which were not reported for palbociclib and ribociclib, and a higher incidence of abdominal pain.22-25 Notably, in September 2019, the FDA warned that rare but severe, life-threatening, or fatal interstitial lung disease (ILD) and pneumonitis can occur in patients treated with CDK4/6 inhibitors.26 Therefore, patients should be monitored regularly for pulmonary symptoms indicative of ILD and/or pneumonitis.
Conclusion
The approvals of palbociclib, ribociclib, and abemaciclib in recent years have expanded treatment options for patients with advanced breast cancer. Although they all possess the same mechanism of action, these drugs have slight differences in efficacy and safety. A number of ongoing studies beyond those mentioned in this review continue to explore these inhibitors in different patient populations and with different treatment combinations. The potential of these agents to expand the use of CDK4/6 inhibitors into settings other than HR+, HER2– advanced breast cancer appears promising.27
REFERENCES
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30.
2. Preusser M, De Mattos-Arruda L, Thill M, et al. CDK4/6 inhibitors in the treatment of patients with breast cancer: summary of a multidisciplinary round-table discussion. ESMO Open. 2018;3(5):e000368.
3. Tripathy D, Bardia A, Sellers WR. Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin Cancer Res. 2017;23(13):3251-3262.
4. Portman N, Alexandrou S, Carson E, et al. Overcoming CDK4/6 inhibitor resistance in ER-positive breast cancer. Endocr Relat Cancer. 2019;26(1):R15-R30.
5. Sobhani N, D’Angelo A, Pittacolo M, et al. Updates on the CDK4/6 inhibitory strategy and combinations in breast cancer. Cells. 2019;8(4):E321.
6. Ibrance (palbociclib) package insert. New York, NY: Pfizer Inc; September 2019.
7. Kisqali (ribociclib) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; September 2019.
8. Verzenio (abemaciclib) package insert. Indianapolis, IN: Eli Lilly and Co; September 2019.
9. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
10. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925-1936.
11. Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719-729.
12. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med. 2015;373(3):209-219.
13. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425-439.
14. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926-1936.
15. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738-1748.
16. Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541-1547.
17. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465-2472.
18. Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514-524.
19. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised, phase 3 trial. Lancet Oncol. 2018;19(7):904-915.
20. Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307-316.
21. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2– metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218-5224.
22. Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2– advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884.
23. Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116-124.
24. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646.
25. Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;17(5):5.
26. FDA. FDA warns about rare but severe lung inflammation with Ibrance, Kisqali, and Verzenio for breast cancer. www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-severe-lung-inflammation-ibrance-kisqali-and-verzenio-breast-cancer. Accessed January 15, 2020.
27. Shah M, Nunes MR, Stearns V. CDK4/6 inhibitors: game changers in the management of hormone receptor–positive advanced breast cancer? Oncology (Williston Park). 2018;32(5):216-222.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






