US Pharm. 2023;48(7):HS2-HS6.
ABSTRACT: An allergy develops when an individual’s immune system has a heightened reaction to an allergen, leading to a variety of common and bothersome symptoms that can range in severity. Allergy symptoms are typically treated with oral, intranasal, or inhaled prescription or nonprescription medications, but severe cases may also necessitate the use of infused allergy treatment. Infused allergy treatment is the parenteral administration (IV, SC, intrathecal, or epidural) of antiallergy medications. A number of different options are available for infused treatment, including monoclonal antibodies and immunoglobulin. Pharmacists can play a key role in screening, medication selection, counseling, and therapy monitoring in patients with allergic conditions whose symptoms may require infused allergy treatment.
Itchy and/or watery eyes, rhinorrhea, sneezing, and rash: These constitute only a handful of the common and bothersome symptoms of allergies. An allergy develops when an individual’s immune system has a heightened reaction to an allergen, a substance that is inert and harmless in nature.1
Upon exposure to an allergen, T helper type 2 (Th2) cells facilitate the production of immunoglobulin E (IgE) antibodies, which then bind to mast cells and basophils.2 Although the initial exposure is usually asymptomatic, subsequent exposures produce signaling cascades that cause the release of histamine, prostaglandins, leukotrienes, and cytokines. As a result, inflammation develops and allergy symptoms occur.1 These symptoms are typically treated with oral, intranasal, or inhaled prescription or nonprescription medications such as antihistamines, corticosteroids, and leukotriene receptor antagonists.3 However, in patients experiencing severe symptoms, allergies may aggravate existing conditions such as asthma, urticaria, atopic dermatitis, and chronic rhinosinusitis, for which these common agents are insufficient. Oral, intranasal, and inhaled agents may not adequately manage the allergy symptoms of patients with these conditions; instead, infused allergy treatment may be necessary to achieve therapeutic efficacy and symptom relief. Infused allergy treatment refers to the parenteral (e.g., IV, SC, intrathecal, or epidural) administration of antiallergy medications. The efficacy of an infused treatment depends on its ability to target the specific immunoglobulins, interleukins (ILs), and cytokines responsible for producing inflammation upon exposure to an allergen.4
Infused allergy treatment helps reduce inflammation, and common therapeutic targets include IL-5 and IgE.4 Pharmacists can take an active role in facilitating treatment by properly screening patients prior to therapy initiation. Other responsibilities include selecting agents based on patients’ risks, needs, and preferences; educating patients on the clinical reasons for infused treatment; counseling on the therapeutic benefits and expected side effects; monitoring; and providing follow-up care. The pharmacist’s role will be discussed in detail in this article.
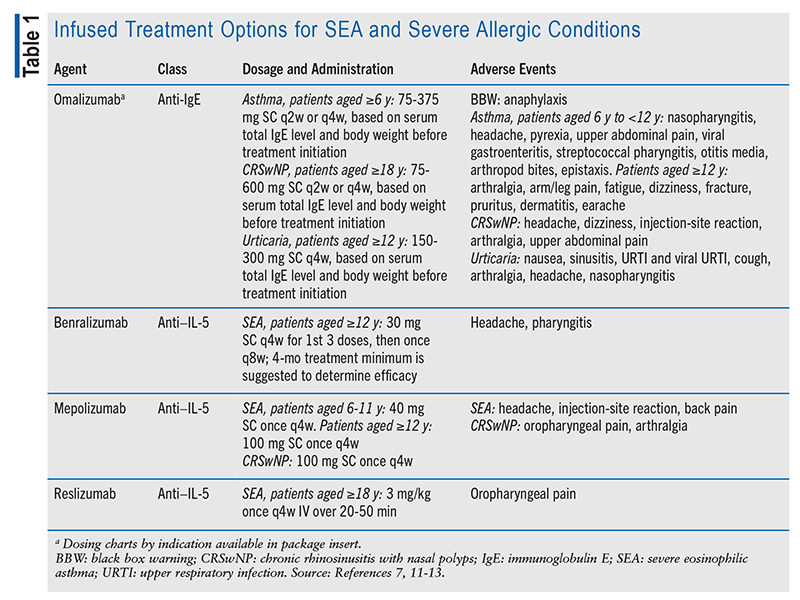
Infused Allergy Treatment Options
A key mediator of allergic reactions is antigen-specific IgE, which binds to high-affinity IgE receptors (FcERIs) on mast cells and sensitizes them upon exposure to an allergen. Binding to the FcERI leads to the degranulation of proteases and vasoactive histamines, cytokine transcription, and the production of eicosanoids, leukotrienes, and prostaglandins.5 Omalizumab, a recombinant humanized monoclonal antibody (mAb), is the first anti-IgE agent to prevent the interaction between free serum IgE and FcERI by binding to IgE. Consequently, mast-cell and basophil degranulation is inhibited and free serum IgE levels decrease, resulting in the downregulation of FcERI surface expression on cells.5
Omalizumab is approved by the FDA as add-on maintenance therapy to combined inhaled corticosteroid and long-acting beta-agonist (ICS/LABA) treatment in patients aged 6 years and older who have severe eosinophilic asthma (SEA).6 This agent is administered SC every 2 to 4 weeks, and the dose and frequency are determined by the patient’s body weight and initial total IgE levels.6 Omalizumab may also be used as adjunctive therapy in patients who have chronic rhinosinusitis with nasal polyps (CRSwNP), an allergic condition characterized by increased production of IgE and eosinophilic inflammation. Similarly, the dose and frequency depend on body weight and total IgE levels prior to treatment initiation, and it is administered every 2 to 4 weeks.7 This agent is also used to treat urticaria, at a dosage of 150 mg or 300 mg. Omalizumab has a black box warning for anaphylaxis, a serious, life-threatening allergic reaction.7 Prior to omalizumab administration, it is imperative to ensure that epinephrine is available nearby in case anaphylaxis occurs.
High-dose IV immunoglobulin (IVIG) is another type of infused allergy treatment. In vitro studies have demonstrated that IVIG can lessen Th2 secretion, thereby decreasing IgE synthesis and reducing inflammation. Multiple studies have demonstrated the benefits of IVIG in managing asthma, atopic dermatitis, and urticaria. In general, the use of IVIG was shown to improve urticaria scores from baseline, reduce flare and wheal presentation, decrease asthma severity, and effect a marked decline in symptom scores for atopic dermatitis.8
Anti–IL-5 therapy is another effective treatment option for SEA patients. Allergen exposure leads to eosinophilic inflammation and increased levels of IL-5 in the bronchial mucosa.9 Excess IL-5 contributes to the development of resistance to inhaled and systemic corticosteroids, so the use of adjunctive biological medications such as omalizumab and anti–IL-5 agents is warranted.10 Medications that target IL-5 or IL-5 receptor alpha (IL-5RA) cause a decline in blood and sputum eosinophilia and asthma exacerbations.10 These agents include the humanized mAbs benralizumab, mepolizumab, and reslizumab. Benralizumab binds to IL-5RA to prevent it from interacting with IL-5, whereas mepolizumab and reslizumab bind directly to IL-5.11-13 All three agents ultimately inhibit IL-5 signaling and eosinophil production and survival. These medications are used as add-on therapy in patients with SEA who have uncontrolled asthma despite taking ICS/LABA, and mepolizumab is also an add-on therapy to nasal corticosteroids in patients with CRSwNP.6,14 For benralizumab, a minimum of 4 months of treatment is suggested to determine its efficacy.
See TABLE 1 for a summary of infused treatment options for SEA and severe allergic conditions.
Screening
Several factors should be considered prior to the use of infused allergy treatment. Pharmacists can screen patients and inform them about what to expect from infused treatment. Patient education on infused allergy treatments should start with connecting the cause of the patient’s allergic reactions and symptoms to the need for the treatment. If the patient’s allergy symptoms are due to a current medication, that medication should be discontinued as soon as possible. Pharmacists can identify and recommend alternative agents, if needed. Thorough evaluation of a patient’s allergy symptoms is required to assess whether infused treatment is appropriate for the patient.
Patients often undergo allergy testing prior to starting infused allergy treatment. There are four different methods of allergy testing: serum, skin-prick, intradermal, and patch. Serum testing is the only method that requires specimen collection; once a blood sample is obtained, it is tested for the indicated antibodies and compared with similar allergens in panels. The sample undergoes analysis via enzyme-linked immunosorbent assay, wherein the serum is added to an antigen and the reaction between IgE and the allergen is observed.15 In skin-prick testing, 1 g/L to 20 g/L of each allergen, a positive control, and a negative control are placed on the patient’s skin, separated from each other by at least 2 cm. A device is used to scratch the skin so that the allergen can pass through the outer layer of the skin. If an allergy is present, a wheal or hive will appear as a result of mast-cell degranulation.15
Patients undergoing intradermal testing have one or more allergens injected under the skin’s surface, along with a positive and a negative control; a resultant wheal of at least 5 mm indicates an allergic reaction.15 Patch testing involves placing an allergen patch on the patient’s skin—usually the back—for 48 hours; once the patch is removed, the clinician waits 15 to 60 minutes before reading the test results.15
If mAbs are part of a patient’s infused treatment, a tuberculosis (TB) test is required in addition to allergy testing. Individuals infected with TB may not be candidates for infused treatment with mAbs. If a pregnant or lactating patient is not achieving allergy relief from conventional therapies, the decision to start infused allergy treatment should be made after carefully weighing the risks and benefits. Omalizumab, benralizumab, mepolizumab, and reslizumab all cross the placenta, but they appear to have no maternal or fetal impact. Additional data are required to ascertain the safety and efficacy of these agents in pregnant and lactating women.16
Administration, Monitoring, and Patient Education
Infused treatment often requires the collaborative support of healthcare professionals. IV therapies such as reslizumab require an office visit for administration. To ensure adherence to infused treatment, it is important to assess whether the patient has the time and resources to make the necessary medical visits or whether therapies that can be self-administered would be more manageable. To help ensure treatment success for self-administered therapies, pharmacists should train patients on proper technique and safe disposal and storage of medications. It is also important to assess the patient’s level of comfort surrounding self-administration. In all cases, challenges related to cost and medication access must be addressed.
A clear understanding of why parenteral treatment is being added to their therapy is essential for patients; this will increase adherence, which ultimately enables the patient to gain the most benefit if the treatment is tolerated. Counseling should include the therapeutic benefits of infused allergy treatment, and treatment-related side effects (both common and severe) they may experience should be fully discussed. Common adverse reactions include injection-site reactions (e.g., swelling, redness), headache, arthralgia, and body pain.7,12 It is important to communicate the low likelihood of rare but severe side effects, such as allergic reactions, worsening of existing conditions, infections, and malignancy. The benefits versus risks of therapy should be discussed to ensure safe use of the medication and the patient’s comfort level with the treatment. To minimize the risk of preventable disease, pharmacists should ascertain that patients are up-to-date with all immunizations prior to starting treatment.
Pharmacists may assist prior to and during the administration of infused medications. After the treatment is administered, the patient should be asked to sit for at least 15 minutes so that they can be observed for an allergic reaction, such as hives, red/swollen skin, or trouble breathing. In such cases, treatment must be discontinued immediately and epinephrine should be administered as soon as possible. Prior to administering any infused treatment medication, healthcare professionals must have at least one dose of epinephrine prepared in case an anaphylactic reaction occurs. If the patient has been prescribed an SC medication that will be taken in an outpatient setting, two prefilled epinephrine pens should be prescribed as well. When counseling patients, pharmacists should discuss the importance of keeping epinephrine nearby when they are self-administering the medication; in addition, patients should be taught how to recognize an anaphylactic reaction and use the emergency epinephrine in the event that anaphylaxis occurs. It is the duty of the pharmacist to ensure that patients keep up-to-date with the expiration dates of all of these medications as well as the epinephrine supply.
Injection-site reactions may be relieved with the use of ice packs, nonprescription oral analgesics such as acetaminophen or ibuprofen, or topical corticosteroids.17 Patients may visit community pharmacies to seek counseling or may need assistance in selecting the right dosage and product based on their age, current chronic medication use, and comorbidities.
Pharmacists’ Overall Impact
Pharmacists can ensure that patients are optimizing their current therapy prior to starting infused allergy treatment by reviewing with them proper techniques, timing, and dosing of oral and inhaled medications. In a prospective observational study exploring the effects of pharmacist demonstration of inhaler technique, the proportion of patients with optimal inhaler technique increased from 24% to 79% after they received detailed training on inhaler use from a pharmacist.18 Pharmacists can also assess patients’ symptoms to determine whether they should be referred for infused allergy treatment. This may entail asking patients about how they would rate their allergy symptoms on a scale of 1 to 10 using a visual analogue scale, what symptoms they are experiencing, what exacerbates the condition, which medications they have taken to alleviate symptoms, and so on.19 With thorough screening, pharmacists can facilitate patients’ access to necessary medical services.
Conclusion
Although allergic conditions are typically treated with oral, intranasal, or inhaled nonprescription and prescription medications, some patients experience severe symptoms that require infused allergy treatment to achieve optimal therapeutic benefits. Pharmacists can screen patients to assess what may be causing their symptoms, determine whether patients are taking their medications properly (including technique), and evaluate patients’ need for adjunctive therapy with infused allergy treatment. It is important for pharmacists to provide counseling and education to help patients understand the need for infused treatment and the therapeutic benefits they may achieve. Infused treatment is still a new modality for allergic conditions, and clinical studies are ongoing to evaluate the efficacy of novel medications in treating SEA, CRSwNP, and urticaria. In the future, as additional agents gain FDA approval for the aforementioned indications, pharmacists can potentially play an integral role in administering infused allergy treatment.
REFERENCES
1. British Society for Immunology. Allergy. www.immunology.org/policy-and-public-affairs/briefings-and-position-statements/allergy. Accessed April 28, 2023.
2. Deo SS, Mistry KJ, Kakade AM, Niphadkar PV. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India. 2010;27(2):66-71.
3. Hossenbaccus L, Linton S, Garvey S, Ellis AK. Towards definitive management of allergic rhinitis: best use of new and established therapies. Allergy Asthma Clin Immunol. 2020;16:39.
4. Nagase H, Ueki S, Fujieda S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol Int. 2020;69(2):178-186.
5. Gauvreau GM, Arm JP, Boulet LP, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J Allergy Clin Immunol. 2016;138(4):1051-1059.
6. Global Initiative for Asthma. Global strategy for asthma management and prevention, 2023. Updated May 2023. www.ginasthma.org. Accessed June 8, 2023.
7. Xolair (omalizumab) product information. South San Francisco, CA: Genentech, Inc; March 2023.
8. Rabinovitch N, Gelfand EW, Leung DY. The role of immunoglobulin therapy in allergic diseases. Allergy. 1999;54(7):662-668.
9. Varricchi G, Bagnasco D, Borriello F, et al. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol. 2016;16(2):186-200.
10. Pelaia C, Paoletti G, Puggioni F, et al. Interleukin-5 in the pathophysiology of severe asthma. Front Physiol. 2019;10:1514.
11. Fasenra (benralizumab) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; February 2021.
12. Nucala (mepolizumab) product information. Durham, NC: GlaxoSmithKline; March 2023.
13. Cinqair (reslizumab) product information. West Chester, PA: Teva Respiratory, LLC; February 2020.
14. Bachert C, Sousa AR, Han JK, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps: treatment efficacy by comorbidity and blood eosinophil count. J Allergy Clin Immunol. 2022;149(5):1711-1721.e6.
15. Birch K, Pearson-Shaver AL. Allergy testing. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
16. Shakuntulla F, Chiarella SE. Safety of biologics for atopic diseases during pregnancy. J Allergy Clin Immunol Pract. 2022;10(12):3149-3155.
17. Thomaidou E, Ramot Y. Injection site reactions with the use of biological agents. Dermatol Ther. 2019;32(2):e12817.
18. Giraud V, Allaert FA, Roche N. Inhaler technique and asthma: feasability and acceptability of training by pharmacists. Respir Med. 2011;105(12):1815-1822.
19. Klimek L, Bergmann KC, Biedermann T, et al. Visual analogue scales (VAS): measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care. Allergo J Int. 2017;26(1):16-24.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






