US Pharm. 2016;41(5):41-45.
ABSTRACT: Pediatric bacterial meningitis is a medical emergency requiring immediate initiation of treatment. Although the United States and other developed countries have seen a decline in pediatric meningitis, bacterial meningitis continues to cause high morbidity and mortality globally. Vaccinations (Haemophilus influenzae type b, pneumococcal, and meningococcal) have significantly reduced the risk of bacterial meningitis in developed countries. The treatment of bacterial meningitis depends on the suspected or known causative organism. Treatment often incorporates a third-generation cephalosporin or penicillin plus vancomycin. Dexamethasone may be added to prevent neurologic sequelae such as hearing loss. Despite aggressive therapy, many patients will experience long-term neurologic complications.
Pediatric bacterial meningitis is a severe, life-threatening infection of the membranes (meninges) surrounding the brain and spinal cord. The infection may be associated with long-term, potentially devastating sequelae even when it is aggressively managed. Compared with viral meningitis, which frequently is self-limiting and has a good prognosis, bacterial meningitis carries a higher risk of morbidity and mortality. According to the CDC, 4,100 cases of bacterial meningitis were reported in the United States from 2003 to 2007, and approximately 500 deaths occurred annually during this period.1 It is estimated that the peak incidence of bacterial meningitis occurs in children younger than 2 months of age and that at least 75% of cases occur in those younger than 5 years of age.2-4
Etiology
There are several causative organisms for meningitis in infants and children (TABLE 1). The etiology depends on factors such as age, immune function and immunization status, and geographical location.2-4 The two most common pathogens in the United States are Streptococcus pneumoniae and Neisseria meningitidis.2-4 Globally, Haemophilus influenzae type b (Hib) is a major cause of meningitis. This is in contrast to the U.S. and other developed countries, where the routine immunization of infants with the Hib-conjugate vaccine has greatly reduced the incidence of Hib-related meningitis.5
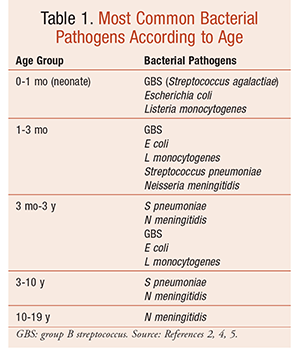
Epidemiology
The global incidence and burden of bacterial meningitis are difficult to determine. Worldwide, the lack of laboratory capacity in certain regions and underreporting lead to a significant variability in incidence.6 The incidence has been reported to be 5 to 10 cases per 100,000 population in high-income countries; however, the incidence also varies with age.5 Population-based surveillance reported 80.69 cases per 100,000 population in patients younger than 2 months of age.4
The incidence of pediatric meningitis has declined substantially with the introduction of vaccines against the three most common bacterial pathogens.2,7,8
Hib: The serotype b of H influenzae is a respiratory pathogen that was once the leading cause of pediatric bacterial meningitis globally. Today, the frequency of Hib in children has declined dramatically with the routine administration of the Hib conjugate polysaccharide vaccine, which was introduced in the 1990s.2
S pneumoniae: The 7-valent pneumococcal conjugate vaccine (PCV) was incorporated into the infant immunization schedule in 2000, and since that time the incidence of pneumococcal meningitis in children in the U.S. has declined by 55% to 60%.9 Despite this, S pneumoniae remains the most frequent cause of bacterial meningitis in children. One reason is that more than 91 distinct serotypes of pneumococcus have been identified.2 In addition, there has been an increase in non-PCV serotypes that cause invasive disease. This led to the development of PCV13 for use in infants and the use of 23-valent polysaccharide vaccine in older children and adults.2
N meningitidis: N meningitidis is a gram-negative diplococcus that is responsible for invasive meningococcal disease. The incidence is bimodal, with an increase in infants younger than 1 year and in teenagers and young adults.10 In the U.S., the serogroups B, C, and Y are involved in bacterial meningitis.2 The incidence of N meningitidis serogroups C and Y bacterial meningitis has decreased because of the routine immunization of 11- to 18-year-olds with the quadrivalent meningococcal glycoconjugate vaccine. Although this vaccine is approved for use in children aged 2 to 10 years, it is not part of the routine immunization schedule. There are concerns with serogroup B N meningitidis meningitis, which currently does not have a vaccine because of its poorly immunogenic capsule.2
Other causative organisms, particularly in infants, include group B streptococcus (GBS) and Listeria monocytogenes. GBS is categorized as early-onset (developing at age <7 days) or late-onset (developing at age >7 days).8 Pregnant women are screened for GBS colonization and, if the patient tests positive, maternal intrapartum antibiotics are initiated during labor to prevent the transmission of GBS to the fetus. The CDC and the American College of Obstetricians and Gynecologists have developed recommendations and guidelines for the prevention of GBS.11,12 L monocytogenes accounted for 3.4% of cases of bacterial meningitis (adults and children) from 1998 to 2007.8 A 36% decline in Listeria occurred during this time period, likely owing to a reduction in food-borne Listeria contamination.8
Pathogenesis and Predisposing Factors
The pathogenesis of pediatric bacterial meningitis is unclear.5 Meningitis-causing pathogens typically cross the blood-brain barrier (BBB) after colonization of the nasopharynx. The mechanism of penetration depends on the organism involved. The BBB also exhibits increased permeability during a meningeal infection. Certain factors increase a person’s risk of bacterial meningitis, including exposure to the infection (i.e., meningococcal) or having a recent upper respiratory tract infection.4
Clinical Features
The clinical presentation of bacterial meningitis is often nonspecific and depends on the patient’s age. Children with meningitis may present with fever and signs of a meningeal inflammation, such as severe and persistent headache, stiff and painful neck (nuchal rigidity), vomiting, and confusion.4,13 The degree of signs and symptoms often depends on the duration of the illness. Some less common symptoms include sluggishness, photophobia, skin rash, and dizzy spells. Petechiae and purpura are most commonly associated with N meningitidis and often begin in the lower extremities.4,5,10
Two clinical signs that may be present in patients with meningitis are Kernig and Brudzinski. A positive Kernig sign is when the patient is lying in the supine position with the hips and knees flexed at 90° and is unable to extend the knee beyond 135° without pain.4 A positive Brudzinski sign is when severe neck stiffness causes the patient’s hips and knees to flex when the neck is flexed.4 Clinicians should keep in mind that the Kernig and Brudzinski signs, as well as nuchal rigidity, are not present in most cases of meningitis in adults and children.13
Generalized seizures occur in approximately 20% to 30% of patients prior to or within 48 hours of admission.14 Although temporary neurologic deficits may occur, these deficits resolve in most patients, and the patients are not at high risk for epilepsy. Patients at high risk for epilepsy are those with permanent neurologic deficits secondary to bacterial meningitis.
Complications
Two types of complications can occur in bacterial meningitis: systemic and neurologic. Systemic complications in children include septic shock, disseminated intravascular coagulation, acute respiratory distress syndrome, and septic or reactive arthritis. Even with treatment, there are significant long-term neurologic effects. At least one in five people with bacterial meningitis has long-term neurologic sequelae.13 Neurologic sequelae include sensorineural hearing loss (reported in 11% of patients), seizures, motor problems, hydrocephalus, and many other cognitive and behavioral problems.4,7 S pneumoniae meningitis has one of the highest rates of long-term complications. The severity of the illness often contributes to the likelihood of long-term neurologic sequelae and higher mortality.2,7 Corticosteroids, which are discussed in the Treatment section, are often administered to lessen the risk of neurologic damage.
Diagnosis
The diagnosis of pediatric bacterial meningitis involves laboratory blood tests and analysis of cerebrospinal fluid (CSF). Blood tests should include a CBC, electrolyte panel, C-reactive protein, and coagulation factors.
Diagnosis relies on CSF analysis. The Infectious Diseases Society of America (IDSA) bacterial meningitis guidelines recommend that lumbar puncture (LP) be performed on children with suspected meningitis.15 There are certain exceptions, including patients who are immunocompromised or have a history of selected neurologic conditions (i.e., CSF shunts, CNS trauma, or neurosurgery).15 In these cases, the patient should first receive a CT scan of the head and have normal findings confirmed prior to LP. The clinician should weigh the risk versus benefit of performing LP in a pediatric patient. Regardless of when the LP occurs, antibiotic treatment should be initiated immediately.15
In addition to the methods mentioned above, a Gram stain of CSF fluid also is performed.15 The Gram stain is positive in about 90% and 80% of children with pneumococcal and meningococcal meningitis, respectively.14 In H influenzae and Listeria meningitis, the Gram stain is positive in about 50% and 30% of patients, respectively. Generally, a patient whose CSF fluid has a high WBC count and a positive Gram stain has a poorer outcome.
Molecular techniques such as polymerase chain reaction may be helpful in the diagnosis and resolution of bacterial meningitis.14,15 A rapid antigen-agglutination test may also be used in diagnosis.13 This option covers a wide range of organisms, such as Neisseria meningococcal serogroups, S pneumoniae, H influenzae, and Escherichia coli, but it is less sensitive. Because false-positive results have been reported with this method, many institutions do not use it.13 The rapid antigen-agglutination test may be most useful in patients who have been pretreated with antimicrobial therapy and whose CSF and Gram stain cultures are negative.
The Bacterial Meningitis Score (BMS) may be used to determine the likelihood of bacterial and nonbacterial meningitis in infants and children older than 2 months.4 Criteria include positive CSF Gram stain, incidence of a seizure with illness, blood neutrophil count exceeding 10,000 cells/mm3, CSF neutrophil count greater than 10,000 cells/mm3, and CSF protein exceeding 80 mg/dL.
Patients at low risk for bacterial meningitis have a score of 0, meaning that they lack the above criteria. A score of 1 point suggests that nonbacterial (aseptic) meningitis is less likely, and a score of >2 suggests that bacterial meningitis is more likely. A meta-analysis reported the BMS has a sensitivity of 99.3% and a specificity of 62.1%.4 The BMS may be helpful for determining the patient’s risk of bacterial meningitis.
Treatment
Principles of Antimicrobial Therapy: Empirical therapy is selected based on the common bacterium causing the meningitis. Once the pathogen is identified, specific treatment may be made based on the known organism. Additional considerations in selecting treatment depend on the drug’s ability to penetrate the BBB. Certain characteristics of antimicrobials that permit easier penetration across the BBB include low molecular weight, simple chemical structure, high lipid solubility, low degree of protein binding, and low degree of ionization. For instance, vancomycin tends to have better penetration when the BBB is significantly damaged. Whether the antibiotic has concentration-dependent (aminoglycosides and fluoroquinolones) or time-dependent (vancomycin and beta-lactams) killing properties should also be considered. Antibiotics should be carefully deescalated once the organism has been identified.2
Antibiotics: Globally, numerous organizations have treatment guidelines for bacterial meningitis, including the IDSA, the Canadian Paediatric Society, the National Institute for Health and Care Excellence, and the Meningitis Research Foundation.15-18 The 2004 IDSA guideline on bacterial meningitis is currently being updated, with publication expected in the autumn of 2016. Most of the recommendations provided here are from the IDSA guideline, although there is minimal variation between the various organizations.
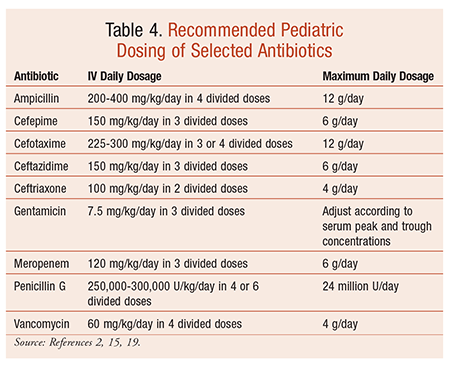
The initiation of empirical therapy for the management of bacterial meningitis should occur immediately following LP or when meningitis is suspected.15 Treatment is on an inpatient basis. Selection of empirical therapy depends on the most prevalent organisms for each age group (TABLES 2 and 3), as well as on local resistance patterns. General recommendations often include a third-generation cephalosporin, such as ceftriaxone or cefotaxime. Ampicillin or penicillin G may be used against susceptible organisms. Pediatric dosing recommendations for selected antibiotics are summarized in TABLE 4.
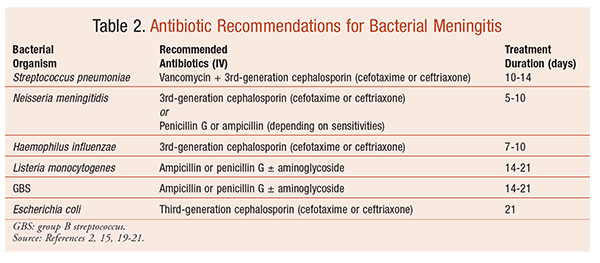

Drug resistance is a concern in the treatment of several organisms. Because of increasing apprehension regarding multidrug-resistant strains of S pneumoniae, penicillin is not recommended for empirical therapy.2,15,19 Instead, vancomycin is often added to the empirical regimen with a third-generation cephalosporin. The American Academy of Pediatrics (AAP) recommends initiating vancomycin with cefotaxime or ceftriaxone in all children aged 1 month and older with suspected meningitis and then deescalating once the organism is identified.20 The IDSA guidelines also recommend the addition of vancomycin whenever S pneumoniae is suspected.15 Rifampin, which has excellent CSF penetration, may be used in cases of cephalosporin-resistant pneumococcal meningitis.2 Penicillin resistance has also been reported to occur with N meningitides; for this reason, penicillins are avoided and third-generation cephalosporins are first-line therapy for meningococcal meningitis.
The duration of treatment has not been fully elucidated. A meta-analysis examining the duration of antibiotics did not find conclusive evidence to support either long or short courses of antibiotics in the treatment of pediatric bacterial meningitis.15,19,21 The current recommendations are based more on experience than on scientific evidence. TABLE 2 lists treatment durations.
Corticosteroids
Despite antibiotic therapy, patients with meningitis are at risk for long-term neurologic complications. There is some evidence that the use of anti-inflammatory agents such as corticosteroids may reduce brain injury.2,15 Corticosteroids decrease inflammation and the release of cytokines, including tumor necrosis factor alpha. Dexamethasone is the most common corticosteroid used to prevent or minimize the neurologic complications of meningitis. The data, however, are conflicting. Initial trials suggested an advantage for dexamethasone used adjunctively to decrease neurologic sequelae (particularly hearing loss), with the greatest benefit in cases of Hib meningitis. A 2015 Cochrane review found limited evidence from two trials that suggested reductions in death and hearing loss with use of adjuvant corticosteroids, and there was no advantage for reducing neurologic sequelae.22
Currently, the IDSA guidelines recommend that adjunctive corticosteroids be used in infants and children with Hib infections, with initiation 10 to 20 minutes prior to the first antibiotic.15 Corticosteroids should not be given to patients who have already received antimicrobial therapy, owing to a lack of benefit. Although the IDSA guidelines do not provide recommendations for pneumococcal meningitis, the AAP suggests that dexamethasone be considered in patients aged 6 weeks and older after weighing the risks and benefits.15 There is no evidence supporting the routine use of adjunctive corticosteroids in meningococcal meningitis.
Several dosing regimens of dexamethasone have been used. The most common dosage is 0.15 mg/kg every 6 hours for 4 days.15,19 Dexamethasone should be given prior to or simultaneously with the first dose of antibiotics. An auditory assessment should be performed at discharge and 1 month afterward.
Other limited agents are being used for the anti-inflammatory treatment of pediatric bacterial meningitis. Glycerol, a hyperosmolar diuretic, has also been used as adjunctive treatment; its low cost and oral administration are advantages, but more data are needed in order to recommend this agent for the prevention of neurologic sequelae.2
Supportive Care
The management of meningitis includes the administration of IV fluids.19,23 The patient’s fluid and electrolyte balance should be assessed. If patient is in shock or hypovolemic, rehydration should be provided. If the patient has an appropriate fluid status, moderate fluid restriction (generally two-thirds or three-fourths of their daily maintenance) should be considered. Pediatric patients with meningitis may have excess total and extracellular water, increased secretion of the antidiuretic hormone, mild systemic hypertension, and increased intracranial pressure. In initiating hydration, it is important to rule out inappropriate secretion of antidiuretic hormone.
Chemoprophylaxis
Chemoprophylaxis may be considered to reduce the risk of person-to-person transmission of meningitis (i.e., siblings and other home contacts).1 Rifampin may be prescribed to close contacts to reduce the transmission of Hib and meningococcal meningitis.1,14 Vaccination is recommended for children who are unvaccinated and eligible for the Hib vaccine. For the meningococcal infection, adults and pediatric patients may receive rifampin or ceftriaxone. Ciprofloxacin 500 mg orally as a single dose is another option for adults, but it is not recommended for pediatric patients owing to the potential for cartilage damage.14 Unvaccinated children and close contacts older than 2 years who have been exposed to meningococcus A, C, Y, or W135 should receive the quadrivalent meningococcal vaccination. Refer to the CDC Immunization Schedules for the most up-to-date recommendations (www.cdc.gov/vaccines/schedules). Chemoprophylaxis is not indicated for pneumococcal meningitis. Generally, healthcare workers do not require chemoprophylaxis.
Conclusion
Pediatric bacterial meningitis is often fatal if treatment is delayed. Pharmacists should be aware of the signs and symptoms to assist in the timely diagnosis of meningitis in pediatric patients. If meningitis is suspected, an examination of the CSF via LP should be performed in eligible patients to determine the organism involved. Immediate initiation of antibiotics and supportive care is essential for reducing the morbidity and mortality of meningitis. Even with treatment, patients may have long-term neurologic sequelae. Pharmacists should recommend the discussed vaccinations knowing that they may reduce the risk of meningitis.
REFERENCES
1. CDC. Bacterial meningitis. www.cdc.gov/meningitis/bacterial.html. Updated April 1, 2014. Accessed January 22, 2016.
2. Agrawal S, Nadel S. Acute bacterial meningitis in infants and children: epidemiology and management. Paediatr Drugs. 2011;13:385-400.
3. de Jonge RC, van Furth AM, Wassenaar M, et al. Predicting sequelae and death after bacterial meningitis in childhood: a systematic review of prognostic studies. BMC Infect Dis. 2010;10:232.
4. Kaplan SL. Bacterial meningitis in children older than one month: clinical features and diagnosis. UpToDate. www.uptodate.com/contents/bacterial-meningitis-in-children-older-than-one-month-clinical-features-and-diagnosis. Accessed February 28, 2016.
5. Heckenberg SG, Brouwer MC, van de Beek D. Bacterial meningitis. In: Aminoff MJ, Boller F, Swaab DF, et al, eds. Handbook of Clinical Neurology. Neurologic Aspects of Systemic Disease. Amsterdam, the Netherlands: Elsevier B.V.; 2014.
6. Luksic I, Mulic R, Falconer R, et al. Estimating global and regional morbidity from acute bacterial meningitis in children: assessment of the evidence. Croat Med J. 2013;54:510-518.
7. Kim KS. Acute bacterial meningitis in infants and children. Lancet Infect Dis. 2010;10:32-42.
8. Thigpen MC, Whitney CG, Messonnier NE, et al. Bacterial meningitis in the United States, 1998-2007. N Engl J Med. 2011;364:2016-2025.
9. Tin Tin Htar M, Madhava H, Balmer P, et al. A review of the impact of pneumococcal polysaccharide conjugate vaccine (7-valent) on pneumococcal meningitis. Adv Ther. 2013;30:748-762.
10. Dwilow R, Fanella S. Invasive meningococcal disease in the 21st century—an update for the clinician. Curr Neurol Neurosci Rep. 2015;15:2.
11. CDC. Group B strep (GBS). www.cdc.gov/groupbstrep. Accessed February 28, 2016.
12. American College of Obstetricians and Gynecologists. Prevention of early-onset Group B streptococcal disease in newborns. www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Prevention-of-Early-Onset-Group-B-Streptococcal-Disease-in-Newborns. Accessed February 28, 2016.
13. El Bashir H, Laundy M, Booy R. Diagnosis and treatment of bacterial meningitis. Arch Dis Child. 2003;88:615-620.
14. Tunkel AR. Meningitis. www.antimicrobe.org/e7.asp. Accessed February 28, 2016.
15. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.
16. National Institute for Health and Care Excellence. Meningitis (bacterial) and meningococcal septicaemia in under 16s: recognition, diagnosis and management. www.nice.org.uk/guidance/cg102/chapter/guidance. Accessed January 22, 2016.
17. Le Saux N. Guidelines for the management of suspected and confirmed bacterial meningitis in Canadian children older than one month of age. Canadian Paediatric Society. www.cps.ca/documents/position/management-of-bacterial-meningitis. Accessed January 22, 2016.
18. Meningitis Research Foundation. Meningococcal meningitis and septicaemia: guidance notes. www.meningitis.org/assets/x/50631. Accessed January 22, 2016.
19. Kaplan SL. Bacterial meningitis in children older than one month: treatment and prognosis. UpToDate. www.uptodate.com/contents/bacterial-meningitis-in-children-older-than-one-month-treatment-and-prognosis. Accessed February 28, 2016.
20. Bamberger DM. Diagnosis, initial management, and prevention of meningitis. Am Fam Physician. 2010;82:1491-1498.
21. Schroeder AR, Ralston SL. Intravenous antibiotic durations for common bacterial infections in children: when is enough enough? J Hosp Med. 2014;9:604-609.
22. Ogunlesi TA, Odigwe CC, Oladapo OT. Adjuvant corticosteroids for reducing death in neonatal bacterial meningitis (review). Cochrane Database Syst Rev. 2015;11:CD010435.
23. Kaplan SL. Bacterial meningitis in children: neurologic complications. UptoDate. www.uptodate.com/contents/bacterial-meningitis-in-children-neurologic-complications. Updated September 8, 2015. Accessed January 23, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.





