US Pharm. 2018;43(9):HS2-HS9.
ABSTRACT: Abnormal uterine bleeding, which affects many women of reproductive age, can result in reduced quality of life, decreased productivity, and an increase in overall healthcare burden. Pharmacotherapy options are first-line, with a focus on combined hormonal contraceptives and levonorgestrel-releasing intrauterine systems. Surgical options, such as dilation and curettage, endometrial ablation, and hysterectomy, should be reserved for patients in whom pharmacotherapy was ineffective or is contraindicated. Pharmacists are useful resources for helping patients recognize signs and symptoms of abnormal uterine bleeding, addressing medication-taking behaviors, and optimizing drug therapy.
In nonpregnant women, deviations in menstrual flow that exceed patient-perceived normal quantity, duration, regularity, or frequency are considered to be abnormal uterine bleeding (AUB).1 AUB, which affects up to 14% of reproductive-age women, can significantly impact quality of life. Mood changes, heightened stress, changes in libido, decreased work productivity, and increased financial burden often result. These variations in the menstrual cycle were previously described as dysfunctional uterine bleeding; however, this term has fallen into disuse.2
Pathophysiology
For the majority of women, a normal menstrual cycle will last 21 days to 35 days, and sometimes even 40 days. Menses typically occurs over 5 days, with an estimated average blood loss of 40 mL.3 The menstrual cycle consists of the follicular phase, which begins on day 1 of menses and lasts until ovulation occurs (about day 14), and the luteal phase, which begins with ovulation and ends at the start of the next menses. The follicular and luteal phases are governed by a feedback loop between the hypothalamus, anterior pituitary, ovaries, and endometrium. Involved in this process are gonadotropin-releasing hormone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH).4-6
Classification
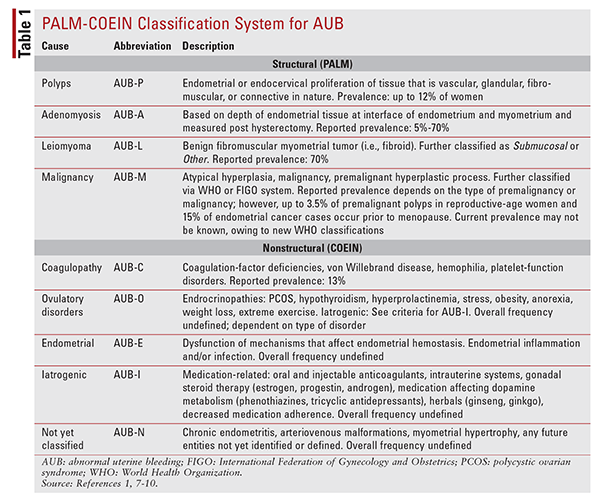
Owing to the variable spectrum of disorders that can cause AUB, a new diagnostic classification system was developed in 2011 by the International Federation of Gynecology and Obstetrics and subsequently adopted by the American College of Obstetricians and Gynecologists (ACOG).1 This widely used classification system, known as PALM-COEIN (polyps, adenomyosis, leiomyoma, malignancy; coagulopathy, ovulatory disorders, endometrial, iatrogenic, not yet classified), classifies AUB based on structural versus nonstructural causes.1
Initially, AUB is divided into two categories. The first category is heavy menstrual bleeding (HMB), which has replaced the term menorrhagia. HMB signifies excessive menstrual bleeding that may reach 80 mL or more. It is important to note that AUB and HMB are based on patient perception of heavy menses and irregularity rather than a specific objective measure. HMB is also a measure of cyclic menses, as opposed to heavy bleeding related to ovulatory dysfunction.1-3 Intermenstrual bleeding (IMB), which replaces the previously used term metrorrhagia, is considered to be any bleeding that occurs outside of clearly defined cyclic menses.1-3 Once AUB has been categorized as HMB, IMB, or both, it is subcategorized into structural causes (PALM) and nonstructural causes (COEIN), as summarized in TABLE 1.1,7-10
To further determine the course of AUB management, it is important to differentiate between acute and chronic AUB. AUB that has been present for 6 months or longer is defined as chronic, whereas a bleeding episode of sufficient quantity to require immediate treatment is defined as acute. Sufficient quantity is a subjective term, and there is no strict definition of acute AUB. Light, normal, and heavy menstrual blood loss averages 5 mL, 40 mL, and ≥80 mL, respectively. Blood loss resulting in hemodynamic instability is considered acute AUB.3 It is possible to develop episodes of acute AUB in the presence of chronic AUB, or the acute episode may occur spontaneously.11
ACOG recommendations are based on three different age groups.2-4 In young women aged 13 to 18 years, AUB is more likely to be related to ovarian dysfunction and anovulation; this is primarily due to the immaturity of the hypothalamic-pituitary-gonadal axis, which affects the hormonal feedback loop.2-4 In women aged 19 to 39 years, AUB may be associated with anovulation (e.g., polycystic ovarian syndrome [PCOS]), pregnancy, medication use, endometrial hyperplasia, and structural abnormalities. In women aged 40 years to menopause, declining ovarian function is the primary cause of AUB; however, it is important to rule out endometrial hyperplasia, carcinoma, and other structural changes.2-4
Patient Assessment
The clinician should obtain detailed information regarding age at menarche, menopausal age (if applicable), patterns of menstruation, bleeding length and severity, pain severity, comorbid medical conditions, past surgical history, current medication regimen, medication-taking behaviors, and signs or symptoms of a possible hemostatic disorder.2,4,12,13 Screening tools for coagulation disorders have been developed.14,15 Further workup is needed in patients with AUB who screen positive for one or more of the following: 1) excessive menstrual bleeding since menarche; 2) history of postpartum hemorrhage, surgery-related bleeding, or bleeding during dental work; or 3) history of two or more episodes of bruising >5 cm one to two times monthly, nosebleeds one to two times monthly, or family history of bleeding symptoms.14,15
Physical assessment should include a pelvic examination and a Pap smear to identify the presence or absence of lesions in the vaginal tract or cervix. The size and contour of the uterus should be determined via bimanual examination. Additionally, a general physical examination includes inspection for signs of PCOS, such as facial hair or acne, and an assessment of presence or absence of skin changes that might indicate the presence of a bleeding disorder.2,4,12
Laboratory screenings include a CBC to assess for anemia and/or coagulopathy, a pregnancy test, a test of prolactin levels, and testing of thyroid-stimulating hormone to detect the presence of hypo- or hyperthyroidism. Cervical cancer screening should be performed, and high-risk patients should be evaluated for the presence of the sexually transmitted infection (STI) Chlamydia trachomatis.2-4
Transvaginal ultrasonography, sonohysterography, hysteroscopy, and MRI are techniques employed to visualize structural abnormalities of the uterus if there are positive physical findings or if management with medication fails.2,4,12
Endometrial sampling via biopsy is recommended for women with a high risk of developing endometrial cancer.4 Women at higher risk include those aged 45 years and older and those with history of one of the following, regardless of age: BMI >30 kg/m2, past use of unopposed estrogen, past or current use of tamoxifen, or family history of endometrial or colon cancer.12 Endometrial sampling may be done on an outpatient basis, and it is considered minimally invasive and less costly compared with inpatient dilation and curettage.4 However, outpatient sampling procedures are limited by a 0% to 54% sample-rate failure. This failure is influenced by uterine and cervical abnormalities, endometrial-cavity size, and the size, type, and quantity of lesions present.4
Medical Management
Management of AUB consists of medication and/or surgical management. The choice is made based on shared decision making between patient and provider to address acute or chronic symptoms.
Acute AUB: For acute AUB, therapy goals include controlling the current bleeding episode, reducing the potential for future episodes, and decreasing blood loss during subsequent menstrual cycles.12 Pharmacotherapeutic management consisting of combined oral contraceptives (COCs), oral progestins, IV conjugated equine estrogens, or tranexamic acid is considered first-line.12 In certain situations, dilation and curettage, endometrial ablation, uterine artery embolization, or hysterectomy may be indicated (TABLE 2).2,4,12,16,17 Acute AUB often requires immediate treatment via pharmacologic or surgical therapy.
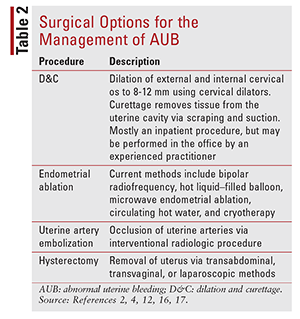
Chronic AUB: Goals of therapy in managing chronic AUB include cycle control, decreased blood loss during menstrual cycles, and prevention of acute AUB episodes. Surgical management is similar to that for acute AUB (TABLE 2). Pharmacotherapeutic management includes combined hormonal contraceptives (CHCs—pill, patch, vaginal ring), oral progestins, tranexamic acid, the levonorgestrel-releasing intrauterine system (LNG-IUS), depot medroxyprogesterone acetate, leuprolide acetate, danazol, mefenamic acid, and ibuprofen.5,11-16,18-21 Pharmacotherapy is preferred over surgical options if the cause of AUB is nonorganic. When to initiate therapy is based on shared decision making between provider and patient.6,10 It is important to identify the cause of chronic AUB prior to therapy initiation. The timing of therapy initiation is variable, and it is based on patient preference if AUB has been present for 6 months or longer in the absence of organic causes.6,10
Pharmacotherapy
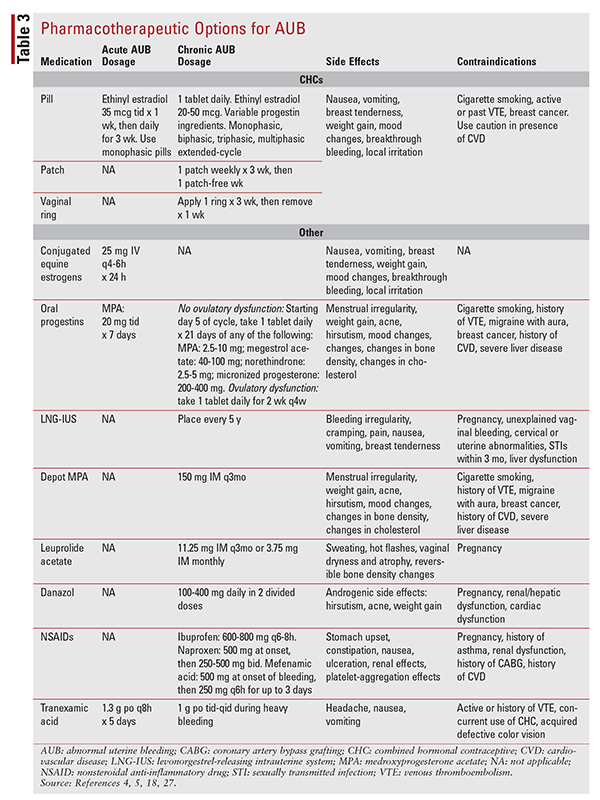
Pharmacotherapeutic options for acute and chronic AUB are summarized in TABLE 3. CHCs, LNG-IUS, progesterone, tranexamic acid, and nonsteroidal anti-inflammatory drugs (NSAIDs) have been shown to reduce menstrual blood flow by 40% to 50%, 79% to 97%, 37% to 87%, 34% to 60%, and 10% to 51%, respectively.22-25 The choice of pharmacotherapy should be guided by shared decision making, presence or absence of comorbid disease states, social history, adherence to therapy, and cost. A 2016 chart published by the CDC that summarizes medical eligibility and recommendations for contraceptive choice based on age, lifestyle, behavior, and comorbidities is a useful assessment tool for medication optimization.26 Currently, only oral CHCs, oral progestins, LNG-IUS, and tranexamic acid are approved for chronic AUB. IV equine estrogens have FDA approval for acute AUB, and leuprolide acetate is approved for treatment of fibroids.6,27,28
There is a paucity of data regarding the use of complementary and alternative medicine (CAM) in the treatment of AUB. Literature supporting the use of these therapies focuses primarily on postmenopausal symptoms. However, it is important for providers to identify whether patients are using CAM in order to monitor for drug interactions, side effects, and changes in AUB symptom presentation.
Therapy effectiveness is based on reduced frequency of AUB episodes, decreased menstrual blood loss per menstrual cycle, improved pelvic pain control, and better quality of life. The presence or absence of medication side effects should be monitored, with therapy adjusted as needed. Laboratory monitoring parameters depend on the choice of drug therapy but may include a CBC and a comprehensive metabolic panel to monitor renal and hepatic function and improvements in anemia (if present). Initial monitoring should take place within 1 to 2 months of therapy initiation, with routine monitoring thereafter.6
CHCs: For acute AUB, COCs containing ethinyl estradiol 35 mcg should be taken three times daily for 7 days. For chronic AUB, any form of monophasic, triphasic, extended, or continuous monophasic COC may be used. A transdermal patch that delivers ethinyl estradiol 35 mcg and norelgestromin 150 mcg daily is available. A third option—the vaginal ring—delivers ethinyl estradiol 15 mcg and etonogestrel 120 mcg daily. With the exception of extended or continuous COCs, each option provides active hormone 21 days per month, with a hormone-free period of 7 days.4,18,19,23 Of the oral COCs, only a four-phase formulation is currently FDA-approved for treatment of AUB; this product contains synthetic estradiol valerate and dienogest.6,22,27,28
Conjugated Equine Estrogens: IV conjugated equine estrogens are indicated for the management of acute AUB and are not used for chronic AUB. This agent is administered at a dosage of 25 mg IV every 4 to 6 hours for 24 hours.11,18
Oral Progestins: Medroxyprogesterone acetate, at a higher dosage of 20 mg three times daily for 7 days, may be used for acute AUB symptoms. For chronic AUB, norethindrone, megestrol acetate, micronized progesterone, or lower doses of medroxyprogesterone acetate (2.5-10 mg) may be used for chronic AUB. Dose and duration are dependent on the presence or absence of ovulatory dysfunction.4,11,18,19,23,29
Tranexamic Acid: Tranexamic acid is an antifibrinolytic agent that decreases blood loss. Only the oral formulation is used for AUB. Rather than affecting the hormonal feedback loop, tranexamic acid exerts its effect by competitively blocking plasminogen binding sites. This lessens the ability of clots and fibrin to degrade and prevents plasma formation. Tranexamic acid may be used for both acute and chronic AUB.4,11,18,29
LNG-IUS: Indicated for the treatment of chronic AUB, the LNG-IUS releases to the endometrium a localized daily dose of levonorgestrel 20 mcg, which has the potential to reduce systemic side effects. A provider office visit is required for placement at 5-year intervals. For patients who have difficulty with adherence to oral medication or who have failed oral therapies, the LNG-IUS might be a viable treatment option.4-6,12,18,29,30
Depot Medroxyprogesterone Acetate: Depot medroxyprogesterone acetate is administered at a dosage of 150 mg IM every 12 weeks. This medication often requires an office visit for administration. Some patients find that AUB symptoms worsen initially and then improve with continued use. Contraceptive studies indicate that amenorrhea may occur within 1 year of continuous use.5,6,18,29
Leuprolide Acetate: Leuprolide acetate is a gonadotropin-releasing hormone that results in atrophy of the endometrial lining. This medication is indicated for chronic AUB and is administered at a dosage of 3.75 mg IM monthly or 11.25 mg IM every 3 months. Side effects often limit the use of this medication.4,5,18,29
Danazol: Danazol creates endometrial atrophy by inhibiting secretion of FSH and LH by the pituitary gland. It is administered in two divided daily doses of 100 mg to 400 mg. Danazol has been shown to be more effective than progestins, NSAIDs, and CHCs in reducing menstrual blood loss.18,29,31 Side effects are primarily androgenic in nature.
NSAIDs: NSAIDs decrease menstrual blood loss by increasing platelet aggregation and vasoconstriction via cyclooxygenase inhibition and active effects on thromboxane A2 and prostaglandins E2 and F2A. Mefenamic acid, ibuprofen, and naproxen are options for the treatment of chronic AUB.4,5,18,19,29
The Pharmacist’s Role
Pharmacists practicing in many areas of pharmacy care may assist with AUB management. In clinics and community practice, pharmacists may help guide pharmacotherapy selection via provider consultation, shared decision making, and collaborative-practice agreements. Patient counseling opportunities include assessment of medication-taking behavior and adherence to drug therapy. Pharmacists can encourage patients to maintain a menstrual diary to collect objective and subjective data regarding menstrual-cycle patterns. Diary information should contain frequency and length of menstrual flow, cramping and pain levels, OTC therapies tried, and mood changes. Patients should be encouraged to keep a log of how many pads or tampons are used, the type used (e.g., super, regular, light), and at what frequency they are changed.
Pharmacists have long been a primary point for patient questions and triage, and they can recommend provider office visits or urgent-care visits when necessary. Additionally, because the causes of AUB may also include the presence of STIs, pharmacists can facilitate appropriate treatment and reporting of STIs. Finally, pharmacists can recommend cost-effective therapies or facilitate therapeutic substitutions based on affordability and formulary options.
REFERENCES
1. Munro MG, Critchley HO, Broder MS, et al. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13.
2. Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
3. Fraser IS, Critchley HO, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29:383-390.
4. Committee on Practice Bulletins—Gynecology. Practice bulletin no. 136: management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
5. Shrader SP, Ragucci KR. Contraception. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill Education; 2017.
6. Umland EM, Klootwyk J. Menstruation-related disorders. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill Education; 2017.
7. Lee SC, Kaunitz AM, Sanchez-Ramos L, Rhatigan RM. The oncogenic potential of endometrial polyps: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:1197-1205.
8. Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstet Gynecol Scand. 2010;89:992-1002.
9. Whitaker L, Critchley HO. Abnormal uterine bleeding. Best Pract Res Clin Obstet Gynaecol. 2016;34:54-65.
10. Ryntz T, Lobo RA. Abnormal uterine bleeding: etiology and management of acute and chronic excessive bleeding. In: Lobo RA, Gershenson DM, Lentz GM, Valea FA, eds. Comprehensive Gynecology. 7th ed. Philadelphia, PA: Elsevier; 2017.
11. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
12. Billow MR, El-Nashar SA. Management of abnormal uterine bleeding with emphasis on alternatives to hysterectomy. Obstet Gynecol Clin N Am. 2016;43:415-430.
13. Sweet MG, Schmidt-Dalton TA, Weiss PM, Madsen KP. Evaluation and management of abnormal uterine bleeding in premenopausal women. Am Fam Physician. 2012;85:35-43.
14. Kouides PA, Conard J, Peyvandi F, et al. Hemostasis and menstruation: appropriate investigation for underlying disorders of hemostasis in women with excessive menstrual bleeding. Fertil Steril. 2005;84:1345-1351.
15. Kadir RA, Lukes AS, Kouides PA, et al. Management of excessive menstrual bleeding in women with hemostatic disorders. Fertil Steril. 2005;84:1352-1359.
16. De La Cruz MS, Buchanan EM. Uterine fibroids: diagnosis and treatment. Am Fam Physician. 2017;95:100-107.
17. Williams VL, Thomas S. Dilation and curettage. In: Pfenninger JL, Fowler GC, eds. Pfenninger & Fowler’s Procedures for Primary Care. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2011.
18. Bradley LD, Gueye NA. The medical management of abnormal uterine bleeding in reproductive-aged women. Am J Obstet Gynecol. 2016;214:31-44.
19. Matteson KA, Rahn DD, Wheeler TL II, et al. Nonsurgical management of heavy menstrual bleeding: a systematic review and practice guidelines. Obstet Gynecol. 2013;121:632-643.
20. Hickey M, Higham JM, Fraser I. Progestogens with or without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst Rev. 2012;(9):CD001895.
21. Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding—a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
22. Bitzer J, Heikenheimo O, Nelson AL, et al. Medical management of heavy menstrual bleeding: a comprehensive review of the literature. Obstet Gynecol Surv. 2015;70:115-130.
23. Committee on Practice Bulletins—Gynecology. Practice bulletin no. 110: noncontraceptive uses of hormonal contraceptives. Obstet Gynecol. 2010;115:206-218.
24. Committee on Practice Bulletins—Gynecology. Practice bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:1173-1175.
25. Leminen H, Hurskainen R. Tranexamic acid for the treatment of heavy menstrual bleeding: efficacy and safety. Int J Womens Health. 2012;4:413-421.
26. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
27. Lexicomp Online. Hudson, OH: Lexi-Comp, Inc; 2018. http://online.lexi.com. Accessed August 9, 2018.
28. Natazia (estradiol valerate and estradiol valerate/dienogest) package insert. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; August 2015.
29. Sripasert I, Pakrashi T, Kimble T, Archer DF. Heavy menstrual bleeding diagnosis and medical management. Contracept Reprod Med. 2017;2:20.
30. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
31. Beaumont H, Augood C, Duckitt K, Lethaby A. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2007;(3):CD001017.
To comment on this article, contact rdavidson@uspharmacist.com.






