While chronic sun exposure ages skin over the years, causing wrinkles and altering pigmentation, it also predisposes an individual to precancerous changes in skin cells.1,2 Ultraviolet (UV) radiation damages skin cells, known as keratinocytes, causing changes in skin texture and color and producing a blotchy appearance and lesions.2 Skin cell damage is cumulative; therefore, the greater the exposure, the greater the risk for skin cancer. Furthermore, an individual’s risk increases if the majority of outdoor exposure occurs in locations and at times of the day where sunlight is most intense.2 When exposure to UV rays—typically from the sun, but also from tanning beds—is frequent or intense, abnormal keratinocytes can develop into what is known as an actinic keratosis.2
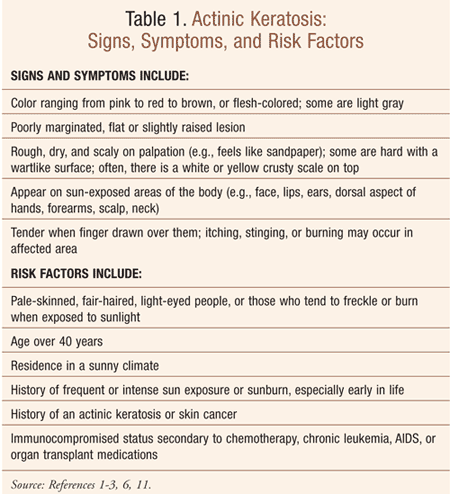
Actinic keratosis, a rough, scaly patch also referred to as a solar keratosis, is commonly found on sun-exposed areas of the skin such as the face, lips, ears, dorsal aspect of the hands, forearms, scalp and neck (TABLE 1). An actinic keratosis takes years to develop and usually makes its appearance in older adults—thus the term senile keratosis—although it can be seen in younger individuals, typically beginning at age 30 or 40 years and becoming more common with advancing age.3
While actinic keratoses are considered to be precancerous changes in the skin that can develop into squamous cell carcinoma, there is no way to determine whether an actinic keratosis will progress to squamous cell carcinoma or how quickly that might happen.1,3,4 Keratoses that occur on the ear and lip are at the highest risk of developing into cancer due to the sensitivity of the ear and lip to sun exposure.3 Pharmacists can help raise awareness of the importance of preventive measures by including them in their patient counseling and providing appropriate guidance. The risk of actinic keratosis can be reduced by minimizing sun exposure and protecting the skin from UV rays.
Signs, Symptoms, Risk Factors, and Diagnosis
TABLE 1 lists signs and symptoms of, and risk factors for, actinic keratosis. Usually more than one actinic keratosis is present, ranging in size from 1 mm to 3 mm (about the size of a small pea) or larger; lesions may be numerous, with several patches close together.3 While typically asymptomatic, lesions may be tender and produce itching, stinging, and burning in the affected area.1,2 While an actinic keratosis can sometimes resolve on its own, it usually recurs after further sun exposure; if scratched or picked off, it will return as well.2
Diagnosis of actinic keratosis is made through a skin examination, which may include the use of a bright light and magnifying lens to inspect the skin and scalp for growths, moles, or lesions.3 Skin biopsy may be taken if there is a suspicion of cancer.3 Actinic keratoses should be differentiated from seborrheic keratoses which increase in size and number as a person ages.1,5 While seborrheic keratoses, can often resemble actinic keratoses, they are distinguished from actinic keratoses upon close inspection as: 1) waxy; 2) seen to be “stuck on”; 3) occurring on non–sun-exposed areas of the body; 4) not premalignant.1
Treatment
Early treatment of actinic keratosis is recommended to halt potential progression to squamous cell carcinoma.3 Choosing a treatment for actinic keratosis from the available options often depends on the number and location of the lesions.1 If lesions persist, they should be evaluated for possible biopsy.6,7 Most commonly, actinic keratoses are treated with liquid nitrogen; topical 5-fluorouracil is another frequently used therapy.4
Topical liquid nitrogen (cryotherapy) is considered a rapid and effective eradication treatment option if only a few actinic keratoses are present; lesions typically blister, peel, crust, and disappear within 10 to 14 days.1,6 Side effects of cryotherapy may include skin texture alteration, infection, and darkening of the skin in the affected area.2
Topical 5-Fluorouracil (5-FU) is chosen if many lesions are present.1 Fluorouracil, an inhibitor of DNA and RNA synthesis, is available in several strengths (0.5%, 2%, 5%) and in both cream and solution formulations.8 It is applied to the lesions once daily at night or twice daily (e.g., in the morning and evening) until they become red and sore at first, then crusted and eroded.1,6,7 The majority of lesions often clear in 2 to 6 weeks, with a typical case taking 2 to 3 weeks.1,6-8 The milder 0.5% cream may provide clearance of lesions with less irritation and therefore may be an appropriate choice for use on the face as compared to stronger concentrations, such as 5% cream, that may be required to eradicate actinic keratosis lesions on the arm.1,6,7
Proper counseling by the pharmacist is necessary regarding the significant reaction produced by 5-FU—erythema, burning, erosion, crusting, and even ulceration, which may be physically uncomfortable and visually unsightly—requiring suspension of treatment for 1 to 3 days in some cases.1,4 Patients should be instructed that masking the reaction with cosmetics and, if necessary, suppressing it with topical corticosteroids are effective.1 Of note, 5-FU should not be used to treat basal cell carcinomas, unless they are of the superficial type as determined by biopsy.1 For a review of the treatment of squamous cell carcinoma and basal cell carcinoma, see Reference 4 and/or Reference 5.
Topical imiquimod, an antineoplastic/immunomodulator, is considered an alternative to topical 5-FU; it is often used for treatment of actinic keratoses and superficial basal cell carcinomas.1 While the exact mechanism is unknown, in essence this agent stimulates the immune system to recognize and destroy cancerous skin lesions.1,8 Imiquimod 5% cream is applied 2 times per week at bedtime for 16 weeks; the treatment area should be limited to 25 cm2 on the face or scalp.8,9 The milder 3.75% cream is applied at bedtime for 2 weeks, followed by no treatment for 2 weeks, then another 2 weeks of treatment.8,9 Of note, the safety and efficacy of imiquimod have not been established in immunocompromised patients.
Topical diclofenac, a phenylacetic acid, is a nonsteroidal anti-inflammatory agent; the 3% gel formulation is applied twice daily for 60 to 90 days. While the precise mechanism is still unknown, it is thought to be related to prostaglandin synthetase inhibition.9 Dermatologic adverse effects include application site reaction, including dermatitis.9
Curettage (scraping) to remove the lesion, and electrosurgery (electrodesiccation) to control bleeding and destroy any remaining abnormal cells may be used; curettage, while a quick treatment, can cause infection, changes in skin pigmentation, and scarring, including the risk of developing a keloid.3 A keloid may cause itching or may progressively enlarge, due to excessive collagen formation in the dermis during connective tissue repair.3,10
Photodynamic therapy uses intense laser light combined with a photosensitizing agent (aminolevulinic acid) to destroy the actinic keratosis.2,3 Side effects may include redness, swelling and a burning sensation during the treatment period.2
Chemical peeling with one or more solutions (e.g., trichloroacetic acid) may be applied to the lesions, causing blistering and eventual peeling that allows new skin to form.2 The skin peeling usually lasts for 5 to 7 days and may also cause stinging, a burning sensation, redness, crusting, changes in skin coloration, infections, and scarring (rarely).2 Since this procedure is often considered cosmetic in nature, it may not be covered by medical insurance.2
Tips for Prevention
Limiting exposure to the sun is helpful in preventing the development and recurrence of actinic keratosis, which, as previously mentioned, is considered a precancerous condition. The following is a synopsis of detailed tips for prevention of actinic keratosis that can be found at the Web site in the accompanying box, Resource for the Patient, and given at WebMD3:
• Limit time exposed to the sun, especially from 10 A . M . to 4 P . M .
• Use sunscreen every day; it should block ultraviolet rays (both UVA and UVB) and have a sun protection factor of at least 15
• Cover up by wearing protective clothing and sunglasses that block UV rays
• Avoid tanning beds, sunlamps, and tan-accelerating agents
• Check skin regularly and report changes for evaluation by a doctor
Note that skin burns faster at higher altitudes, and where sun exposure is stronger in or near surfaces that reflect light, such as water, sand, concrete, and areas painted white.11

Conclusion
Pharmacists have a role in the identification, treatment, and prevention of actinic keratosis. Becoming familiar with the risk factors and physical characteristics of actinic keratosis lesions, including signs and symptoms, is just a start. Provision of thorough counseling regarding significant treatment reactions and outcomes, and detailed prevention techniques, is an integral component of the pharmaceutical care of patients with this precancerous condition.
REFERENCES
1. Chronic effects of sunlight.
Merckmanuals.com. Revised August 2007.
www.merckmanuals.com/professional/dermatologic_disorders/reactions_to_sunlight/chronic_effects_of_sunlight.html?qt=actinic
keratoses&alt=sh#v961876. Accessed March 19, 2012.
2. Actinic keratosis. Mayoclinic.com. Published January 7, 2011. www.mayoclinic.com/health/actinic-keratosis/DS00568. Accessed March 19, 2012.
3. Actinic keratosis. Topic overview. WebMD.
Updated October 1, 2010.
www.webmd.com/skin-problems-and-treatments/tc/actinic-keratosis-topic-overview.
Accessed March 19, 2012.
4. Cheigh NH. Dermatologic drug reactions and self-treatable skin disorders. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York, NY: McGraw-Hill Inc; 2008:1584-1585.
5. Porter RS, Kaplan JL eds. The Merck Manual of Diagnosis and Therapy. 19th ed. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 2011:746,748-754.
6. Berger TG. Dermatologic disorders. In: McPhee SJ, Papadakis MA, Rabow MW, eds.
Current Medical Diagnosis & Treatment 2011. 50th ed. McGraw Hill Medical; New York,NY. 2011:119.
7. McIntyre WJ, Downs MR, Bedwell SA. Treatment options for actinic keratoses. Am Fam Physician. 2007;76(5):667-671.
8. Epocrates, Version 4.1. Epocrates, Inc.
www.epocrates.com. Updated March 19, 2012.
9. Micromedex, Version 1.24.0b1060. www.Micromedex.com. Accessed January 12, 2012.
10. Dorland’s Pocket Medical Dictionary. 28th ed. Philadelphia, PA: Elsevier Saunders: 2009.
11. Actinic keratosis. Medline Plus.
U.S. National Library of Medicine. National Institutes of Health.
Updated November 22, 2011.
www.nlm.nih.gov/medlineplus/ency/article/000827.htm. Accessed March 19,
2012.
To comment on this article, contact rdavidson@uspharmacist.com.





