US Pharm. 2012;37(12):HS1-HS8.
ABSTRACT: As drug experts, pharmacists are looked upon to provide appropriate guidance for the treatment of acute bacterial peritonitis in the hospital setting. Acute bacterial peritonitis is associated with a high risk of mortality. Immediate intervention is necessary, as delay can allow the once-localized disease process to damage other organs throughout the body. Appropriate identification and treatment of acute bacterial peritonitis are keys to better outcomes. In addition to surgical intervention, pharmacologic treatment is also necessary. Treatment or prevention of hypovolemia associated with peritonitis may be carried out per the recommendations of the Surviving Sepsis Campaign guidelines. Finally, since antibiotic therapy is the third cornerstone of treatment, it is important to consider the appropriate selection, dosing, and duration of antibiotics.
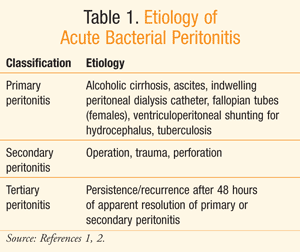
Peritonitis is an acute inflammation of the peritoneal lining due to bacterial infection as well as other causes such as chemicals, irradiation, and foreign-body injury.1 Insult of the peritoneal lining by any of these agents can lead to an inflammatory response, known as acute peritonitis.1 TABLE 1 displays common pathogenic etiology of acute bacterial peritonitis.
Mortality rates associated with peritonitis were around 90% in the early 1900s. These rates have since come down to approximately 30% with the use of appropriate drug therapies and supportive care.2
Disease Overview
Classification of bacterial peritonitis is based upon the source of the infectious bacteria.2 Primary or spontaneous peritonitis refers to an extraperitoneal etiology, in which the infectious bacteria enter the peritoneal cavity through the circulatory or lymphatic system.1,2 In these cases, the patient usually has an underlying comorbidity that can lead to bacterial migration into the peritoneum.1 Such comorbidities may include ascites and indwelling peritoneal dialysis catheters. Primary peritonitis is estimated to occur in 10% to 30% of patients with alcoholic cirrhosis.1 Additionally, patients on chronic ambulatory peritoneal dialysis (CAPD) have, on average, one incidence of peritonitis every 33 months.1
Secondary peritonitis, the most common etiology, is the result of infectious bacteria from a source within the peritoneum.1 Considering the plethora of microflora existing within the abdominal organs, migration of the bacteria from any of the organs into the sterile peritoneum can lead to an inflammatory response, resulting in secondary peritonitis. Dispersion of bacteria from their host organs may result from puncture due to trauma, surgery, or perforation.1 Ulceration, ischemia, or obstruction may cause the perforation of abdominal organs.1 Finally, tertiary peritonitis is persistent or recurrent peritonitis that reappears at least 48 hours after apparent resolution of a primary or secondary peritonitis.2 Data on the incidence of secondary and tertiary peritonitis are limited.1 Classification of peritonitis is useful in clinical practice as it can help facilitate appropriate diagnosis and treatment.
Clinical Presentation and Diagnosis
Bacterial introduction into the peritoneum results in an immediate humoral and cellular immune response.1 This response triggers an inflammatory process, which results in fluid shifts into the peritoneal cavity. This fluid accumulation, along with decreased intestinal motility, often leads to abdominal distention. Additionally, fluid displacement into the abdomen, known as third-spacing, may result in decreased blood volume, leading to hypovolemia in severe cases. Fever, vomiting, and diarrhea may also accompany peritonitis, compounding fluid imbalances and increasing the risk for hypovolemia. Untreated hypovolemia can result in decreased cardiac output, and, ultimately, hypovolemic shock.1
In addition to fluid shifts, foreign substances such as feces and mucus present in the abdominal cavity may worsen peritonitis by weakening immune mechanisms such as bacterial phagocytosis.1 Bacterial and endotoxin absorption into the bloodstream is facilitated by an inflamed peritoneum and may result in sepsis. Death may result from negative effects on organ systems from serious complications such as sepsis. Additionally, other serious complications, such as hypoalbuminemia caused by protein loss as well as pulmonary complications like pneumonia resulting from diaphragmatic inhibition due to splinting, may worsen the prognosis. Along with host immunity and treatment adequacy, the amount and virulence of infecting organisms and the presence of foreign substances within the abdominal cavity have a great impact on the resulting outcome of peritonitis.1
The nonspecific clinical presentation of primary peritonitis varies drastically from the conspicuous presentation of secondary peritonitis.1 Unlike secondary peritonitis, primary peritonitis may develop over several weeks without any signs of acute distress. Although the patient may complain of abdominal tenderness, nausea, vomiting, or diarrhea, primary peritonitis is usually first suspected when the dialysate appears cloudy in patients undergoing peritoneal dialysis or when encephalopathy worsens in patients with cirrhosis. The laboratory findings following suspicion of primary peritonitis may show mild elevation of the white blood cell (WBC) count and a positive culture of the peritoneal dialysate or ascetic fluid.1
In contrast, patients with secondary peritonitis often present with a boardlike abdomen, abdominal distention, faint bowel sounds that diminish over time, and excruciating abdominal pain that leads to involuntary guarding, with minute movements such as breathing or rocking of the bed causing severe pain. As previously mentioned, hypovolemia may occur in the absence of treatment, which may ultimately result in hypotension and shock. Other signs and symptoms include tachypnea, tachycardia, nausea and vomiting, decreased urine output, and elevated temperature.1 In secondary peritonitis, laboratory tests may show leukocytosis, with predominating neutrophils and elevated bands.1
Treatment of Peritonitis
The desired outcomes in peritonitis include resolution of the underlying etiology and drainage of abscesses.1 Secondary goals of treatment include elimination of infection and protection from adverse drug events as well as end-organ damage, including that to the lungs, liver, heart, and kidneys.1 Elements of appropriate intervention consist of fluid resuscitation, source control procedure (surgery), and empirical antimicrobial therapy.3 Most cases of primary peritonitis require the use of antimicrobial agents as the mainstay of therapy, and drainage procedures usually are not required.1
Secondary peritonitis requires surgical treatment, known as source control, to correct the underlying pathology.1 Source control intends to correct anatomical derangements, remove infectious foci, and control the factors promoting ongoing infection.4 Specific factors that are likely to preclude a successful source control include a delay of more than 24 hours until the procedure; an Acute Physiology and Chronic Health Evaluation (APACHE) score of at least 15; age >70 years; presence of comorbidity; low albumin level; poor nutritional status; diffuse peritonitis; and presence of malignancy.3 Failed source control is indicated by recurrent infection at the site, anastomotic failure, or fistula formation.3 Percutaneous, image-guided drainage is preferred over surgical drainage, especially when the infection is well localized.1,3
Hypovolemia in the setting of peritonitis can lead to organ failure. Therefore, regardless of the presence of septic shock, rapid fluid resuscitation is warranted in all patients with peritonitis in order to promote physiological stability.3 More aggressive restoration of intravascular volume should be provided to those with septic shock and organ failure in the manner described in the Surviving Sepsis Campaign guidelines for managing septic shock.3 The 2012 updates to the Surviving Sepsis guidelines will reportedly state that the initial fluid challenge should be at least 1 L of crystalloid, and a minimum of 30 mL/kg in the first 4 to 6 hours. Incremental fluid boluses can follow as long as the patient’s vital signs continue to show improvement.5 Norepinephrine is the preferred vasopressor, and dobutamine inotropic therapy can be added on to patients with cardiac dysfunction.5 Continuous hydrocortisone infusion totaling 200 mg/24 hours is recommended for those with vasopressor-refractory septic shock. Fever, tachypnea, nausea and vomiting, and reduced fluid intake can lead to dehydration in patients with peritonitis.3
Empirical antibiotic therapy should be initiated upon suspicion of peritonitis.3 Practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that empiric antimicrobial therapy be initiated within the first hour of the recognition of peritonitis in patients with compromised hemodynamic or organ function; otherwise, therapy should be initiated within 8 hours of presentation.3 Although there is limited value to blood cultures in the treatment of community-acquired peritonitis, Gram stains to detect the presence of gram-positive cocci or yeast are warranted in high-risk individuals as well as in those with health care–acquired peritonitis.3 Antibiotic therapy can be altered upon results of culture and sensitivity data. Initially, however, IV empiric antibiotic selection should target likely organisms present at the site from which peritonitis was derived, as displayed in TABLE 2.1
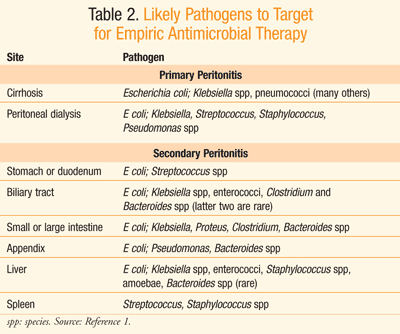
There are differences between health care–associated and community-associated infections (TABLE 3).3,6 Aminoglycosides are among the agents recommended for use in empirical antimicrobial therapy for health care–associated complicated intra-abdominal infections, particularly those caused by Enterobacteriaceae organisms that produce extended-spectrum beta-lactamases (ESBLs).3 Vancomycin is recommended in the same situation when methicillin-resistant Staphylococcus aureus (MRSA) is the causative organism of the health care–associated infection.3 The origination of the infection in terms of anatomical site as well as setting (i.e., hospital or community) helps guide selection of the most reasonable empirical antibiotic therapy.
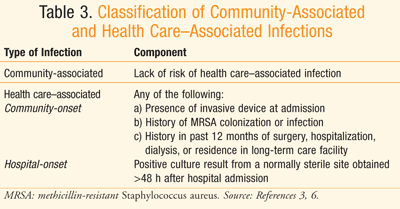
TABLE 4 displays the recommended empirical antibiotic regimens for patients with peritonitis who have normal renal and hepatic function3,6 or a creatinine clearance between 70 and 100 mg/dL.7 Considering the increasing resistance of Escherichia coli to quinolones, such drugs should only be utilized in a hospital whose survey indicates >90% susceptibility of E coli to quinolones.3 There is also concern over the frequent use of ertapenem leading to emergence of carbapenem-resistant Enterobacteriaceae organisms and Pseudomonas and Acinetobacter species.3 Ampicillin-sulbactam should be avoided when targeting E coli, considering the prevalence of resistance to the drug.3,8 When a health care–associated infection is of concern, there is a higher risk of infection due to Candida and Enterococcus species, Fluconazole is warranted in patients who have a malignancy, transplant, inflammatory disease, recurrent intra-abdominal infection, gastric ulcer on acid suppression, or are on immunosuppressive therapy for neoplasm.3 Ampicillin or vancomycin can be added to empiric therapy to target enterococci in those with health care–associated infection, especially when it is a postoperative infection.3 Patients known to be colonized with MRSA and those with health care–associated peritonitis should be treated with an antimicrobial that covers MRSA, such as vancomycin.3
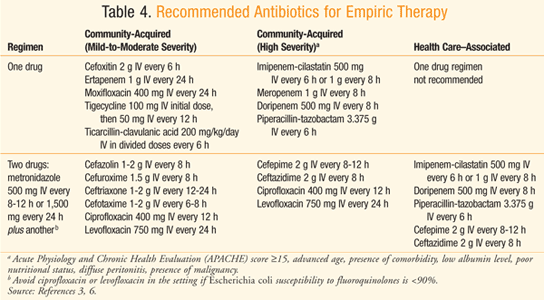
There are specific antimicrobial dosage considerations for patients who are critically ill and/or obese.9 Due to physiological alterations in these patients, certain pharmacokinetic factors may be changed, such as apparent volume of distribution and/or clearance.3 Additionally, if the dosage is dependent on renal function, creatinine clearance may have to be directly measured due to the difficulty of estimation attributable to above-average body weight.3 Critically ill patients may also experience alterations in certain pharmacokinetic parameters. For example, patients in the early stages of sepsis may experience a hypermetabolic state and fluid shifts that can cause increased volume of distribution and clearance.3 Such shifts may result in lower serum concentrations of antimicrobials such as beta-lactams.3 On a similar note, reduced serum concentrations of antimicrobials such as cephalosporins and carbapenems have been observed in obese patients.3 As a result of such pharmacokinetic changes, adjustments such as higher doses and/or more frequent administration may be required in these subgroups of patients.3 In cases where vancomycin is an appropriate agent for critically ill and/or obese patients, total body weight should be used in calculating the initial dosage.3 Additionally, these patients should have serum vancomycin concentration monitored in order to provide individualized dosing.3,10
Intraperitoneal dosing is preferred over IV dosing for patients on peritoneal dialysis to provide higher local levels of antibiotics. Empirical therapy should cover both gram-positive and gram-negative organisms known to cause peritonitis. Intraperitoneal antibiotics can be given through continuous dosing or intermittent dosing. Dosing regimens should taken into account residual renal function, defined as >100 mL/day urine output, in which case recommended doses should be empirically increased by 25%.11 Dwell-time exchange should be at least 6 hours if using intermittent dosing. The recommended duration of treatment is 2 weeks, or 3 weeks for more severe cases. Treatment is dependent on clinical response, which should be seen within 72 hours of initiation of antibiotic therapy. Patients with cloudy effluent after 4 to 5 days of appropriate antibiotic therapy are thought to have refractory peritonitis and should have their catheter removed.11
Acute renal failure is the most important predictor of death among patients with spontaneous bacterial peritonitis (SBP).12 Albumin has been used in SBP to cause plasma volume expansion in order to decrease the incidence of renal failure in patients with cirrhosis undergoing large-volume paracentesis.13 A study evaluating the use of IV albumin in addition to antimicrobial therapy versus antimicrobial therapy alone in patients with cirrhosis and SBP resulted in a decreased incidence of renal failure and decreased mortality.13 It was found that patients who were treated were most likely to benefit from albumin if they had serum bilirubin levels >4 mg/dL, serum creatinine >1 mg/dL, and a blood urea nitrogen (BUN) concentration >30 mg/dL.13 A second study confirmed the previously reported findings and used the same dosing of 1.5 g/kg administered on the first day and 1 g/kg administered on the third day.14 Presently, there are not enough data to support the use of albumin in patients with complicated SBP, or with a serum bilirubin <4 mg/dL and a creatinine of <1 mg/dL. The European Association for the Study of the Liver states that additional studies are needed to assess the efficacy of albumin and other volume expanders in the management of SBP.15
Antimicrobial therapy should be limited to 4 to 7 days.3 If signs and symptoms of peritonitis have resolved at this time, antibiotics are no longer recommended.3 If the patient is recovering at this time, can tolerate an oral diet, and does not demonstrate resistance, step-down therapy with oral antibiotics is warranted.3 Antibiotics recommended in this setting include moxifloxacin, a combination of metronidazole with either levofloxacin or an oral cephalosporin, or amoxicillin-clavulanate. These oral agents can also be used for those who are treated in the outpatient setting but were initiated on inpatient IV therapy.3
Treatment failure after 4 to 7 days of therapy should be investigated through appropriate imaging techniques such as CT scan or ultrasound.3 Antimicrobial therapy effective against initial organisms should be continued and extra-abdominal sources of infection should be ruled out in patients who are nonresponsive to therapy. Aerobic and anaerobic cultures are recommended for patients with infection remaining after initial treatment.3
Certain patient populations should be considered for prophylaxis of bacterial peritonitis. Primary bacterial peritonitis can be prevented using antibiotics if there is a known risk factor. For example, a single IV dose of vancomycin 1 g given at the time of catheter placement in patients undergoing peritoneal dialysis can help prevent bacterial peritonitis.11 An alternate to vancomycin is a single dose of cefazolin 1 g IV.11 Secondary bacterial peritonitis prophylaxis should be considered in patients with cirrhosis who are admitted for upper GI hemorrhage.12 In the past, norfloxacin 400 mg daily had been the drug of choice in the setting of upper GI hemorrhage; however, alternative antimicrobials have since been considered due to epidemiological changes of bacterial infections in cirrhosis.12 Ceftriaxone 1 g IV has been proven to be an effective alternative.16
Conclusion
Peritonitis, an acute inflammation of the peritoneum, can occur due to pathogens or other causes such as chemical exposure. Clinical presentation of primary peritonitis is often nonspecific and may lack initial signs and symptoms. Conversely, secondary peritonitis often presents with prominent symptoms, including severe pain. Untreated peritonitis may result in sepsis and end-organ damage; therefore, prompt treatment of bacterial peritonitis should be guided by specific etiology and comorbidities.
Common antimicrobials of choice for community-acquired peritonitis include cephalosporins and fluoroquinolones. Health care–acquired peritonitis may require treatment with broad-spectrum antimicrobials such as carbapenems. Patient characteristics must be considered in dosing decisions, and certain patient subgroups such as the critically ill and/or obese may require dosage adjustment due to variations in pharmacokinetic parameters. In cases of nonresponse to empirical therapy, sources of infection outside of the abdomen should be considered. In all cases of peritonitis, supportive care should be administered as indicated in order to minimize complications.
REFERENCES
1. Dipiro JT, Howdieshell TR. Intraabdominal Infections. In: Dipiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill; 2011.
2. Ordonez CA, Carlos J. Management of peritonitis in the critically ill patient. Surg Clin North Am. 2006;86:1323-1349.
3. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis
and management of complicated intra-abdominal infection in adults and
children: guidelines by the Surgical Infection Society and the
Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133-164.
4. Marshall JC, Maier RV, Jimenez M, Dellinger EP. Source
control in the management of severe sepsis and septic shock: an
evidence-based review. Crit Care Med. 2004;32(suppl):S513-S526.
5. Friswell A. Surviving Sepsis Campaign previews updated guidelines for 2012. Pulmonary Rev. 2012;17:1,5. www.pulmonaryreviews.com/Article.aspx?ArticleId=sC3JHu+0hSA=. Accessed September 7, 2012.
6. Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;208:1763-1771.
7. Rybak MJ, Lomaestro BM, Rotschafer JC, et al.
Vancomycin therapeutic guidelines: a summary of consensus
recommendations from the Infectious Diseases Society of America, the
American Society of Health-System Pharmacists, and the Society of
Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325-327.
8. Scheetz MH, Hurt KM, Noskin GA, Oliphant CM. Applying antimicrobial pharmacodynamics to resistant gram-negative pathogens. Am J Health Syst Pharm. 2006;63:1346-1360.
9. Murphy JE, Gillespie DE, Bateman CV. Predictability of
vancomycin trough concentrations using seven approaches for estimating
pharmacokinetic parameters. Am J Health Syst Pharm. 2006;63:2365-2370.
10. Owens RC Jr, Shorr AF. Rational dosing of antimicrobial agents: pharmacokinetic and pharmacodynamic strategies. Am J Health Syst Pharm. 2009;66(suppl 4):S23-S30.
11. Piraino B, Bailie GR, Bernardini J, et al. Peritoneal dialysis-related infection recommendations: 2005 update. Perit Dial Int. 2005;25:107-131.
12. Alaniz C, Regal RE. Spontaneous bacterial peritonitis: a review of treatment options. P T. 2009;34:204-210.
13. Sort P, Navasa M, Arroyo V, et al. Effect of
intravenous albumin on renal impairment and mortality in patients with
cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409.
14. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
15. EASL clinical practice guidelines on the management of
ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in
cirrhosis. J Hepatol. 2010;53:397-417.
16. Fernandez J, Ruiz del Arbol L, Gomez C, et al.
Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients
with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049-1056.
To comment on this article, contact rdavidson@uspharmacist.com.





