US Pharm. 2023;48(2):8-12.
ABSTRACT: Anticoagulants are effective for preventing and treating thromboembolic disorders. However, these medications are associated with risk of bleeding complications that can lead to hospitalizations and death. Community pharmacists are well positioned to improve outcomes for patients on anticoagulants by identifying and addressing medication-related problems, as well as providing patient education.
The use of anticoagulants, such as vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs), have continued to rise in the United States as the population grows older.1 While these medications are useful for preventing and treating thromboembolic disorders, they can put the patient at risk for bleeding complications. The VKA warfarin still serves as the standard treatment for patients exhibiting mechanical valve replacements.2 However, for indications such as stroke prevention in atrial fibrillation, or for those with a history of venous thromboembolism (VTE), DOACs are recommended over VKAs.3,4 Similarly effective and generally associated with less bleeding than warfarin, DOACs are increasingly favored due to their convenience. Dosing for warfarin is highly patient-specific and requires routine laboratory monitoring, which may pose a challenge for patients. Compared with warfarin, DOACs have fewer drug interactions and minimal dietary concerns. However, considerations for recommending warfarin over DOACs include severe renal impairment, antiphospholipid syndrome, cost, and compliance.4 If patients are noncompliant with daily use of DOACs, the risk of a thromboembolic event increases due to their short half-lives.5
Potential Medication Errors With Anticoagulants
Medication errors involving anticoagulants can potentially lead to harmful outcomes such as thrombotic events or bleeding, which can contribute to morbidity and mortality.6 These can include dosing errors, especially with warfarin since directions may be complex and frequently change based on the international normalized ratio (INR). If multiple active prescriptions for different warfarin strengths remain on file, a warfarin prescription that was previously discontinued by the prescriber may be inappropriately dispensed. For the DOACs apixaban and rivaroxaban, patients may incorrectly continue the initiation dosage instead of transitioning to the maintenance dose when used for VTE treatment. Apixaban and rivaroxaban should also be reduced to the prophylactic dosage for VTE recurrence after 6 months of standard therapy. Patients on DOACs may also be dosed inappropriately based on their renal function and concomitant medications. Another medication error that can occur with DOACs is unnecessary bridging. Due to their immediate onset and offset, DOACs do not require perioperative bridging. However, when initiating dabigatran or edoxaban for VTE treatment, overlap with an injectable anticoagulant such as low-molecular-weight heparin is acceptable for at least 5 days.7,8 Lastly, inappropriate concomitant use of warfarin and DOACs or multiple DOACs may occur, especially when transitioning anticoagulants due to insurance changes.9
Drug Interactions
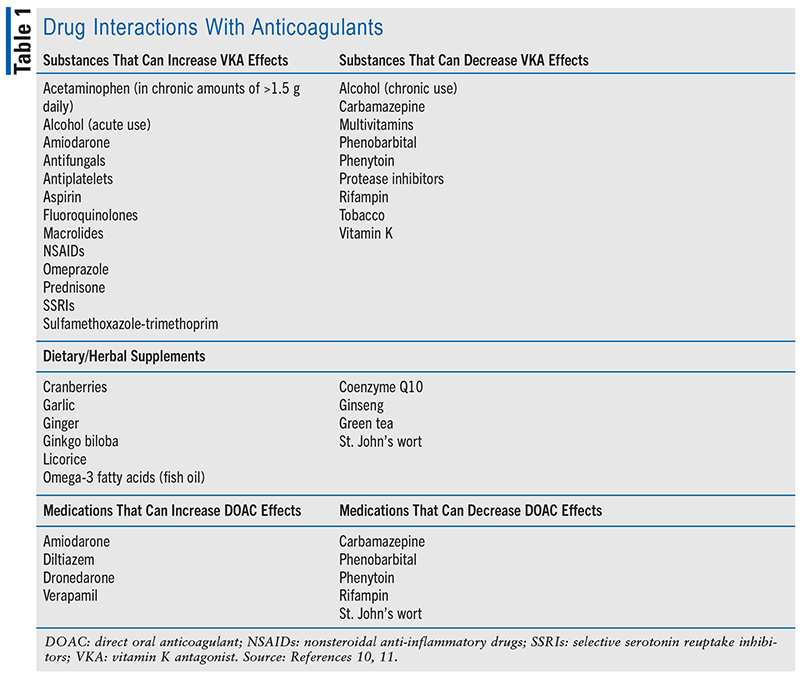
As previously mentioned, one of the challenges with using anticoagulants is managing drug interactions. Not only are prescription drugs a concern but OTC products and complementary medications are as well. TABLE 1 lists common anticoagulant interactions that pharmacists can help identify.10,11 Generally, inhibitors of CYP 2C9 and 3A4 will enhance the anticoagulation effects of VKAs, and inducers will diminish their effects. Patients will respond variably to all types of interactions, but pharmacists can be proactive about managing them. They can suggest therapeutic alternatives with less to no potential for interactions or recommend dosage adjustments—particularly with VKAs—ahead of the expected interactions, if known. Dosage adjustments can range anywhere from 10% to 20% of the patient’s current warfarin dosing in anticipation of an expected interaction. Close INR monitoring is warranted as well. As with warfarin, DOACs can be affected by potent inhibitors or inducers of CYP 3A4.12 Specifically, concentrations of DOACs can be increased by strong inhibitors of CYP 3A4 and P-glycoprotein (P-gp) and decreased by strong inducers of CYP 3A4 and P-gp. Drug interactions may also be more severe when a patient’s renal function is suboptimal.
Patient Education
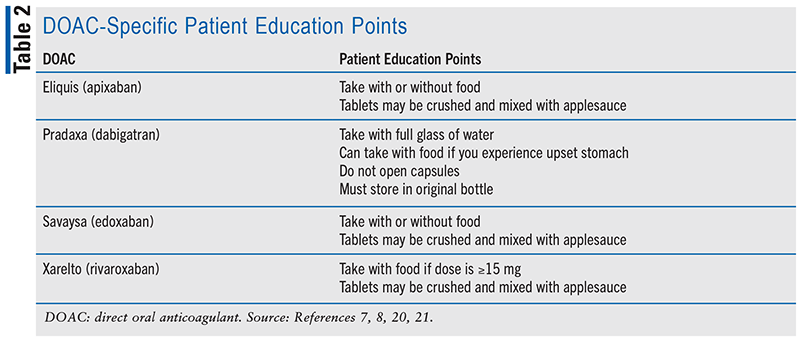
Since suboptimal use of anticoagulants can result in poor outcomes, a pharmacist in any setting can provide a significant amount of education to ensure effective and safe use of anticoagulants. Education and regular reinforcement can promote adherence and identification of medication-related problems (MRPs). For patients on warfarin, these efforts can even result in higher time in therapeutic range (TTR) and, thus, better anticoagulation control.13-16 It can also result in fewer hospital readmissions and lower treatment costs.17 Key education points for anticoagulants can include but are not limited to their indication(s) for use, their mechanism of action, dosing, adherence and missed dose instructions, OTC and prescription drug interactions, lifestyle considerations, monitoring, signs and symptoms of bleeding and thrombosis, management of side effects, and plans for procedures.18,19 For patients on warfarin, pharmacists can also provide education about INR goals and monitoring, as well as food and alcohol interactions. Patient education points regarding administration and storage of DOACs are outlined in TABLE 2.7,8,20,21 Education for anticoagulants should also be tailored to the patient’s needs and literacy levels, as limited health literacy has been associated with suboptimal anticoagulation control.22,23
Pharmacists can help triage managing signs and symptoms of bleeding, as pharmacists in the community setting are often the first healthcare professionals whom patients will contact regarding their concerns. If patients are struggling with regular nosebleeds, nonpharmacologic recommendations include moisturizers (e.g., nasal saline or petroleum jelly) and cool-mist humidifiers. If a patient is suffering from a nosebleed lasting more than 10 minutes, an OTC nasal decongestant like oxymetazoline can be recommended to minimize the nosebleed. Pharmacists can also prevent patients from purchasing interacting OTCs and supplements if they are aware that patients are using anticoagulants.
Community pharmacists are in a prime position to conduct check-ins with patients on anticoagulants, especially since they are regularly picking up refills and other medications at the pharmacy. Particularly useful according to one study was the importance of patients carrying alert cards, which served to communicate with all healthcare providers that someone was using an anticoagulant.24 In cases when patients did not have an alert card, the pharmacist provided education about the importance of having one. Per the study, patients who specifically carried alert cards had greater knowledge about their medication.
Cost-Saving Strategies
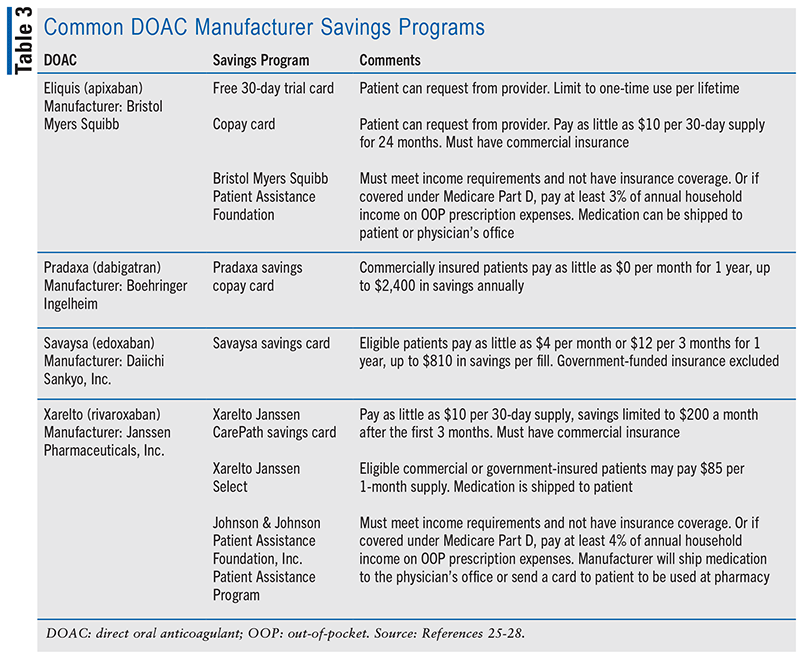
Warfarin is an affordable anticoagulant, especially for patients who are uninsured or underinsured. Even with insurance coverage, patients may still have trouble paying for DOACs, which can negatively affect their adherence to the medication. Pharmacists can help eligible patients lower the cost of their prescriptions by directing them to manufacturer savings programs outlined in TABLE 3.25-28 In 2020, the FDA approved the first generic version of Eliquis (apixaban).29 While the lower cost of generic apixaban can help increase patient access to apixaban, it will not be commercially available until the extended patent for Eliquis expires in 2026.
Community Pharmacy–Based Anticoagulation Models
Pharmacist-led anticoagulation services within ambulatory clinics have been shown to improve patient outcomes, including achieving higher TTRs and lower adverse effects compared with usual care.30 Several studies evaluating anticoagulation services in community pharmacies within the U.S. are available.31,32 These pharmacies utilized point-of-care INR testing waived under the Clinical Laboratory Improvement Amendments of 1988 (CLIA). The pharmacies themselves were also registered as a laboratory through CLIA. In one model, a collaborative practice agreement (CPA) was signed between the referring physician and clinical pharmacist, allowing the pharmacist to monitor patients and adjust warfarin doses based on the INR.32 For the pharmacy that did not have a CPA, the pharmacists contacted the physician and provided dosing recommendations.31 Progress notes were sent to the physician for sign-off and returned to the pharmacy. These studies demonstrated that the majority of patients managed by community pharmacists had INRs in therapeutic range at least 60% of the time, which was comparable with pharmacist anticoagulation services in other settings.31,32
Conclusion
Community pharmacists serve an important role in ensuring safe anticoagulant use. They are well positioned to identify and address MRPs including drug interactions, improve adherence, and provide patient education and guidance on cost-saving resources. Community pharmacists who can establish anticoagulation services can further their ability to monitor and manage anticoagulants, which can help improve patient outcomes.
REFERENCES
1. Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128(12):1300-1305.e2.2. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195.
3. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125-e151.4. Stevens SM, Woller SC, Baumann Kreuziger L, et al. Executive summary: antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160(6):2247-2259.
5. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41(1):206-232.6. Barr D, Epps QJ. Direct oral anticoagulants: a review of common medication errors. J Thromb Thrombolysis. 2019;47(1):146-154.
7. Pradaxa (dabigatran) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; July 2020.8. Savaysa (Edoxaban) package insert. Basking Ridge, NJ: Daiichi Sankyo Inc; April 2021.
9. ISMP. Duplicate oral anticoagulants. ISMP Medication Safety Alert! Community/Ambulatory Edition. 2018;17(3.1).10. Nutescu E, Chuatrisorn I, Hellenbart E. Drug and dietary interactions of warfarin and novel oral anticoagulants: an update. J Thromb Thrombolysis. 2011;31(3):326-343.
11. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e44S-e88s.12. Wiggins BS, Dixon DL, Neyens RR, et al. Select drug-drug interactions with direct oral anticoagulants: JACC review topic of the week. J Am Coll Cardiol. 2020;75(11):1341-1350.
13. Mifsud EM, Wirth F, Camilleri L, et al. Pharmacist-led medicine use review in community pharmacy for patients on warfarin. Int J Clin Pharm. 2019;41(3):741-750.14. Falamić S, Lucijanić M, Hadžiabdić MO, et al. Pharmacist’s interventions improve time in therapeutic range of elderly rural patients on warfarin therapy: a randomized trial. Int J Clin Pharm. 2018;40(5):1078-1085.
15. Cao H, Wu T, Chen W, et al. The effect of warfarin knowledge on anticoagulation control among patients with heart valve replacement. Int J Clin Pharm. 2020;42(3):861-870.16. Tang EOYL, Lai CSM, Lee KKC, et al. Relationship between patients’ warfarin knowledge and anticoagulation control. Annals Pharmacother. 2003;37(1):34-39.
17. Brunetti L, Lee SM, Doherty N, et al. Impact of warfarin discharge education program on hospital readmission and treatment costs. Int J Clin Pharm. 2018;40(3):721-729.18. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e152S-e1845.
19. Hawes EM. Patient education on oral anticoagulation. Pharmacy (Basel). 2018;6(2):34.20. Eliquis (apixaban) package insert. Princeton, NJ: Bristol-Myers Squibb Company; April 2001.
21. Xarelto (rivaroxaban) package insert. Titusville, NJ: Janssen Pharmaceuticals Inc; January 2022.22. Seliverstov I. Practical management approaches to anticoagulation non-compliance, health literacy, and limited English proficiency in the outpatient clinic setting. J Thromb Thrombolysis. 2011;31(3):321-325.
23. Oramasionwu CU, Bailey SC, Duffey KE, et al. The association of health literacy with time in therapeutic range for patients on warfarin therapy. J Health Commun. 2014;19Suppl2:19-28.24. Jani YH, Hirani B, Livingstone C. Evaluation of patients’ knowledge about oral anticoagulant medicines and use of alert cards by community pharmacists. Int J Clin Pharm. 2021;43(1):203-211.
25. Bristol Myers Squibb. Savings & support: Eliquis prescription coverage. www.eliquis.bmscustomerconnect.com/afib/savings-and-support. Accessed November 30, 2022.26. Boehringer Ingelheim. Savings & support: Pradaxa savings. https://patient.boehringer-ingelheim.com/us/pradaxa/pradaxa-savings. Accessed November 30, 2022.
27. Daiichi Sankyo, Inc. The Savaysa savings card. https://savaysa.com/savaysa-support. Accessed November 30, 2022.28. Janssen CarePath. Paying for Xarelto. January 2, 2023. https://www.janssencarepath.com/patient/xarelto/cost-support. Accessed January 11, 2023.
29. FDA. FDA approves first generics of Eliquis. www.fda.gov/news-events/press-announcements/fda-approves-first-generics-eliquis. Accessed November 30, 2022.30. Manzoor BS, Cheng WH, Lee JC, et al. Quality of pharmacist-managed anticoagulation therapy in long-term ambulatory settings: a systematic review. Ann Pharmacother. 2017;51(12):1122-1137.
31. Knowlton CH, Thomas OV, Williamson A, et al. Establishing community pharmacy-based anticoagulation education and monitoring programs. J Am Pharm Assoc (Wash). 1999;39(3):368-374.32. Amruso NA. Ability of clinical pharmacists in a community pharmacy setting to manage anticoagulation therapy. J Am Pharm Assoc (2003). 2004;44(4):467-471.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






