US Pharm. 2021;46(5):9-12.
Diabetes, a prevalent metabolic disease, is characterized by elevated levels of glucose in the blood (i.e., hyperglycemia).1 This chronic condition is caused by the dysfunctioning of the pancreatic beta cells, which are responsible for the production and secretion of insulin. The most common forms of diabetes are type 1, type 2, and gestational diabetes.2
According to the CDC’s National Diabetes Statistics Report, 2020, approximately 34.2 million people in the United States have diabetes, and another 88 million people have prediabetes.3 This translates to roughly one in every 10 persons for diabetes and one in every three persons for prediabetes. The incidence of new diabetes cases among U.S. adults aged 18 years and older is higher in Hispanic and non-Hispanic black persons than in Asian and non-Hispanic white persons.3 Because of the increased prevalence of both prediabetes and diabetes in the U.S. population, it is essential that individuals be regularly evaluated for the presence of diabetes.
Diagnosis
A1C testing is the standard method for analyzing blood glucose (BG) levels over a period of 3 to 6 months. This is because glycosylated hemoglobin forms when glucose irreversibly binds to hemoglobin A1. An increase in glucose level signifies increased binding, which indicates the average glucose concentration that erythrocytes were exposed to over their life span (2-3 months).4,5 Other screening measures for diabetes or prediabetes include fasting plasma glucose and the oral glucose tolerance test (OGTT).2 Two abnormal test results, from either the same sample or different samples, are needed to confirm diagnosis. The presence of classic symptoms of hyperglycemia or hyperglycemic crisis and an elevated random plasma glucose level are also essential for confirming diabetes.2 According to the 2020 guidelines from the American Diabetes Association (ADA), specific diagnostic criteria for diabetes include A1C 6.5% or higher; fasting plasma glucose 126 mg/dL or higher; 2-hour plasma glucose 200 mg/dL or higher after 75-g OGTT; and random plasma glucose 200 mg/dL or higher plus classic symptoms of hyperglycemia (i.e., polyphagia, polyuria, polydipsia) or hyperglycemic crisis.2
Monitoring
The monitoring of glycemic control is a particularly important part of diabetes management, as it prevents life-threatening consequences such as macrovascular and microvascular complications (e.g., cardiovascular disease, renal disease, retinopathy, and/or neuropathy) and assists in the attainment of individualized glucose targets.6,7 Self-monitoring of BG (SMBG) has been used for several decades to track BG levels throughout the day. SMBG has aided patients and healthcare providers in achieving glycemic control, preventing hypoglycemia, and optimizing medication regimens.6-8 Another strategy, continuous glucose monitoring (CGM), is employed primarily in patients with type 1 diabetes but is also an option for patients with type 2 diabetes. CGM automatically tracks BG readings and trends to assess the effectiveness and safety of treatment.6,7,9
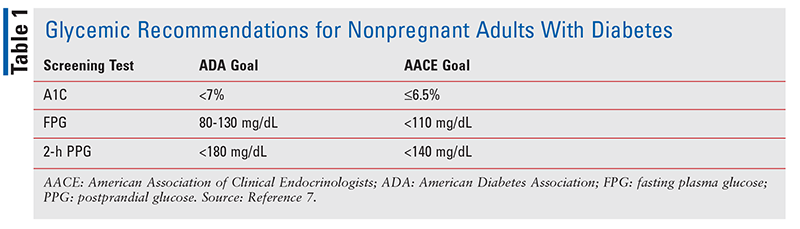
Although SMBG and CGM play pivotal roles in diabetes management, the gold standard for assessing glycemic control is A1C.6 The ADA recommends that A1C measurement be performed every 6 months in patients meeting the treatment goal; however, in patients unable to meet the treatment goal, A1C should be measured every 3 months.6 TABLE 1 outlines glycemic recommendations for nonpregnant adults with diabetes.
Afinion HbA1c Dx Test
Afinion HbA1c Dx (FIGURE 1) is the first rapid point-of-care test (POCT) for determining a patient’s A1C in order to facilitate the diagnosis of type 2 diabetes.10,11 This assay provides accurate results in several minutes, enabling the physician to make a diagnosis immediately.11 Consequently, the patient is able to begin pharmacologic treatment promptly instead of waiting weeks for a confirmed diagnosis. This benefits the patient’s overall health because an earlier diagnosis may confer a reduced risk of developing the microvascular and macrovascular complications of diabetes, resulting in a better prognosis.10,11

The Afinion HbA1c Dx kit includes 15 test cartridges (these are individually packed in foil pouches with a desiccant bag) and a package insert. Other materials that are not supplied but are necessary for use with the kit include the Alere Afinion AS100 Analyzer or the Afinion 2 Analyzer, the Afinion User Manual and Afinion HbA1c Dx Quick Guide (supplied with the analyzer), the Afinion HbA1c Control, and standard blood-collection equipment.12 TABLE 2 summarizes the testing procedure for the POCT.

A group of investigators prospectively assessed the accuracy and precision of the Afinion HbA1c Dx POCT. Blood samples (via finger stick and venous whole blood) were collected from 120 subjects. The investigators found that the POCT was accurate across its measurement range and was highly correlated with the National Glycohemoglobin Standardization Program’s secondary reference laboratory values.13 Both the finger-stick samples and the venous whole-blood samples had Pearson coefficients of at least 0.99. The mean bias for the finger-stick samples and venous whole-blood samples was –0.021% A1C (–0.346% relative bias [RB]) and –0.005% A1C (–0.093% RB), respectively. The 95% CIs of the estimated bias for both the finger-stick and venous whole-blood samples contained the value of 0, signifying the absence of RB or differential bias. The imprecision of the finger-stick and venous whole-blood samples ranged from 0.62% to 1.93% component of variance (CoV) and 1.11% to 1.69% CoV, respectively.13 The Afinion HbA1c Dx POCT demonstrated accuracy and precision with both finger-stick and venous whole-blood samples, and its performance was comparable to that for laboratory testing.10,13
The Afinion HbA1c Dx POCT has some limitations that healthcare providers should keep in mind. The manufacturer specifies that the Afinion HbA1c Dx POCT should not be used for diagnosing diabetes during pregnancy or in certain patient populations.12 These populations include, but are not limited to, diabetes during pregnancy, patients with an elevated fetal Hb (HbF; >10%), such as hereditary persistence of HbF; patients with a hemoglobinopathy but normal red cell turnover; patients with abnormal RBC turnover; and patients with iron-deficiency and hemolytic anemia, various hemoglobinopathies, thalassemias, hereditary spherocytosis, malignancies, and severe chronic renal or hepatic disease. The test also should not be employed in patients who have received a blood transfusion or chemotherapy within the past 3 weeks. Samples that are diluted, coagulated, or hemolyzed cannot be used with the Afinion HbA1c Dx POCT.12
Conclusion
Afinion HbA1c Dx is the first rapid POCT approved for use in diagnosing diabetes and identifying patients at risk for developing diabetes. This POCT has a faster turnaround time for diagnosis, providing accurate results in approximately 3 minutes compared with the several weeks necessary with standard laboratory testing. The precision and accuracy of the Afinion HbA1c Dx assay permit the initiation of diabetes treatment plans earlier than with standard practice, leading to better patient outcomes and increased patient satisfaction. For more information, contact Abbott Diagnostics Technologies AS at (866) 216-9505 or visit the Afinion HbA1c Dx website.11
REFERENCES
1. National Institute of Diabetes and Digestive and Kidney Diseases. What is diabetes? www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes. Accessed April 22, 2021.
2. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes–2020. Diabetes Care. 2020;43(suppl 1):S14-S31.
3. CDC. National Diabetes Statistics Report, 2020. Atlanta, GA: CDC, U.S. Dept of Health and Human Services; 2020.
4. Sacks DB. Measurement of hemoglobin A(1c): a new twist on the path to harmony. Diabetes Care. 2012;35(12):2674-2680.
5. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394.
6. American Diabetes Association. 6. Glycemic targets: Standards of Medical Care in Diabetes–2020. Diabetes Care. 2020;43(suppl 1):S66-S76.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2020 Executive Summary. Endocr Pract. 2020;26(1):107-139.
8. Benjamin EM. Self-monitoring of blood glucose: the basics. Clin Diabetes. 2002;20(1):45-47.
9. Funtanilla VD, Caliendo T, Hilas O. Continuous glucose monitoring: a review of available systems. P T. 2019;44(9):550-553.
10. Foerster V, Severn M. Point-of-care glycated hemoglobin testing to diagnose type 2 diabetes. In: CADTH Issues in Emerging Health Technologies. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2016:156.
11. Abbott. Afinion HbA1c Dx. www.globalpointofcare.abbott/en/product-details/afinion-hba1c-dx.html. Accessed April 22, 2021.
12. Afinion HbA1C Dx package insert. Oslo, Norway: Abbott Diagnostic Technologies AS; October 2020.
13. Arnold WD, Kupfer K, Little RR, et al. Accuracy and precision of a point-of-care HbA1c test. J Diabetes Sci Technol. 2020;14(5):883-889.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





