US Pharm. 2021;46(7):HS1-HS6.
ABSTRACT: The appropriate selection of empiric antimicrobials for the management of aspiration pneumonia can be challenging for providers. Studies detailing the likely bacterial etiology of aspiration pneumonia span more than 40 years and are sufficiently contradictory to cause significant confusion regarding optimal drug therapy. Recommendations from recently published guidelines provide some elucidation, but available literature and the evidence on which these recommendations are based suggest that more research is needed. In the meantime, the clinical pharmacist has an opportunity to impact the use of overly broad antimicrobials while improving therapy for patients who are diagnosed with aspiration pneumonia.
The goal of an antimicrobial stewardship program (ASP) is to promote the safe, effective, and efficient use of antimicrobials in order to optimize patient care while mitigating not only individual patient risks (e.g., secondary infections and antimicrobial-related adverse events) but also the impact of antimicrobial use on the greater population. The ability of an ASP pharmacist to provide interventions and recommendations rests primarily on the foundation of evidence-based clinical guidelines and supporting literature. In instances where the literature is confounding or lacking and long-standing practice is based on poorly evidenced or outdated evidence-based models, it may be difficult to make an impact on antibiotic prescribing habits.
One such instance is the treatment of aspiration pneumonia. Although this condition is considered the leading cause of mortality in patients with dysphagia secondary to neurologic disorders, its bacterial etiology and optimal antimicrobial selection have been debated for more than 40 years.1 The 2019 American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA) updated guideline on diagnosis and treatment of community-acquired pneumonia (CAP) suggests that anaerobic coverage (beyond that of standard recommended CAP regimens) not be routinely added for suspected aspiration pneumonia unless lung abscess or empyema is suspected; this suggestion is conditional based on the very low quality of evidence.2 It is important to recognize that this recommendation may not be effective for all situations in which infection due to aspiration is presumed, and that interpatient variables must be assessed before it can be determined that the use of broader or anaerobic coverage is unwarranted.
Background
Pneumonia is a lower-respiratory-tract infection that involves the parenchyma of the lung. It most commonly occurs as a result of aspiration of an inoculum of pathogens (viruses, bacteria, or, less commonly, fungi) from the oropharynx; inhalation of airborne droplets; or, less commonly, translocation from distal sites of infection via hematogenous spread or contiguous spread from adjacent infected anatomical structures.3 For the purposes of this article, the discussion will be limited to pneumonias whose cause is bacterial and therefore may respond to antibiotic treatment.
Once invasion of the lung parenchyma has occurred, a combination of factors—including the virulence of the infecting organism, the magnitude of the inoculum, and the status of the host’s defenses, as well as the patient’s overall health status—will determine whether the organisms are cleared or if, and to what extent, pneumonia will develop.3
The time to presentation, symptoms, and severity of pneumonia will vary depending on individual patient factors and the causative pathogen(s). Clinical presentation may include cough (with or without production of sputum), fever, chills, shortness of breath, fatigue, altered mentation, and pleuritic chest pain upon inspiration or cough.3,4 Demonstration of an infiltrate by chest x-ray or computed tomography is generally considered a prerequisite for the diagnosis of pneumonia in the hospitalized patient.2,5,6 Additionally, markers such as leukocytosis, blood urea nitrogen, and thrombocytopenia have shown some associative benefit in the diagnosis or severity grading of pneumonia.2,7,8
Pneumonias are generally classified based on a number of factors, including patient disposition (e.g., CAP vs. hospital-acquired pneumonia [HAP]) and situations that may potentiate infection (e.g., ventilator-associated pneumonia [VAP]).2,3,9 Historically, these classifications have suggested a predilection for specific causative organisms, such as Streptococcus pneumoniae in CAP and Pseudomonas aeruginosa in VAP or HAP. Several factors, including but not limited to 1) the development and common use of broad-spectrum oral antibiotics in the outpatient setting; 2) increased use of immune-modifying drugs for a broad set of indications; and 3) the use of antibiotics in the food supply, may contribute to increased antibiotic-resistant bacterial heterogeneity of the community setting.9,10 Another example would be atypical pneumonia, which refers to a pneumonia whose symptoms, radiography, and response to treatment diverge from other pneumonias owing to a more distinct set of causative pathogens (Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Legionella pneumophila).11
Although, as noted earlier, the majority of pneumonias develop from aspiration of colonizing bacteria from the oropharynx, the term “aspiration pneumonia” refers to an infection caused by an aspiration event in a subset of patients under specific circumstances, involving a set of bacterial pathogens that may (or may not) require antimicrobial coverage beyond that of other pneumonia types.1,12 It is best to think of aspiration pneumonia not as a distinct classification of pneumonia, but rather as an extension of CAP and HAP.1,13 It has been estimated that from 5% to 15% of all CAPs are aspiration pneumonias, but because of the lack of specific markers and inconsistency of witnessed aspiration events, it is difficult to know the true number.1,12 Accepting this paradigm of a continuum of pneumonia renders the selection of appropriate antimicrobial therapy more straightforward.
Risk Factors
It has been shown that nearly one-half of healthy adults microaspirate oropharyngeal secretions as they sleep but rarely develop clinically relevant signs of pneumonia.14 By contrast, aspiration pneumonia may readily occur with large-volume aspiration (macroaspiration) of oropharyngeal or upper-gastrointestinal contents harboring colonizing pathogens.12
Additional factors that contribute to the development of pneumonia after an aspiration event include the patient’s disposition at the time of aspiration (community setting vs. healthcare setting), the virulence and inoculum size of aspirated pathogens, and anatomical and/or physiological impairments.1,12-14
As discussed earlier, community-acquired bacteria are—at least historically—somewhat distinct from the bacteria usually acquired in the healthcare setting, where the pooling of infected patients in relatively close proximity and the concentrated use of broad-spectrum antibiotics may select drug-resistant isolates.2,15 Although the most consistent marker for risk of infection with drug-resistant pathogens appears to be the receipt of IV antibiotics, exposure to this pooled set of resistant hospital pathogens promotes oropharyngeal colonization.2,16,17 As expected, this suggests different empiric antibiotic coverage needs in patients who macroaspirate in the community versus those who macroaspirate in the hospital or nursing home setting.12
Patient-specific factors that facilitate aspiration pneumonia include impaired swallowing and impaired ability to clear aspirated contents and pathogens.12 Factors that increase the risk of macroaspiration may include dysphagia (as seen with neurologic deficits); cancers of the head, neck, and esophagus; altered mental status (related to several factors, such as hypoglycemia, prescription medications, alcohol, and illicit substances); seizures; and post resuscitation of cardiac arrest, among other causes.1,12,13
Neurologic conditions that lead to dysphagia include cerebrovascular accident (CVA), dementia, multiple sclerosis, and Parkinson’s disease. Dysphagia and cough impairment associated with CVA will depend on the severity of the damage and the anatomical structures involved.18
Alcohol intoxication can induce respiratory depression and decrease the clearance of foreign matter and bacteria by impairing respiratory cilia. Similarly, opioids and medications administered for the treatment of agitation, delirium, depression, and dementia have been associated with an increase in aspiration pneumonia.13,19,20
Bacteriology
The bacteriology of aspiration pneumonia has been a subject of interest and debate for more than 40 years, with studies from the 1970s that aimed to determine the microbiological etiology still influencing current practice patterns. These early studies, which used transtracheal sampling, suggested that anaerobic bacteria were the predominant cause of aspiration pneumonia either alone or as part of a polymicrobial process, and they became the basis for employing antibiotics with broad anaerobic activity as the treatment standard. Notably, the primary anaerobes isolated in one study were Bacteroides melaninogenicus (now Prevotella melaninogenica) and Fusobacterium nucleatum, both found as colonizers and commensals of the upper airway and the human oral cavity, respectively.21-24
Infections due to anaerobic bacteria often develop slowly because of the less virulent nature of some organisms, and patients may not present with clinically relevant symptoms until late in the infectious process.12 Some critics have postulated that early studies were biased toward isolating anaerobic pathogens, as samples were taken late in the clinical course, when conditions favoring anaerobes (empyemas and abscesses) had developed. Also, patient selection in these studies was noted to be overly inclusive of chronic alcohol abusers and potentially biased toward patients who reported putrid sputum.1,21,22
Two studies from the 1990s that employed a protected specimen brush to sample the lower respiratory tract in patients with diagnosed acute aspiration syndromes suggested that the predominant organism type depended on the patient’s location at the time of the aspiration event. Patients from the community more commonly presented with S pneumoniae, Staphylococcus aureus, Haemophilus influenzae, and Enterobacteriaceae, whereas hospitalized patients were more likely to present with P aeruginosa and other gram-negative organisms. Neither study demonstrated a significant presence of anaerobic organisms despite the use of anaerobic culturing techniques.25,26
In a 2005 study that investigated the microbiology of pulmonary abscesses, Klebsiella pneumoniae was the most prevalent bacterium, followed by the abscess-forming Streptococcus anginosus (Streptococcus milleri) group. Anaerobes were isolated in only 20% of evaluated cases, and patients with anaerobic involvement were more likely to have a prediagnosis symptom duration greater than 30 days, further supporting the belief that anaerobic involvement is less common in acute aspiration events.13,27
Other studies have evaluated the microbiology of aspiration pneumonia; regardless, it appears that—whether due to patient-selection bias in earlier studies or changes in living conditions and healthcare practices (fluoridated water supply, greater healthcare access, etc.)—understanding of the bacteriologic causes of aspiration pneumonia has undergone a significant shift over the past 40 years. It is also evident that close scrutiny of individual patient factors is required when antimicrobial therapy is being selected, rather than approaching treatment as a “one size fits all” model. Available literature and the evidence on which these recommendations are based suggest that more research is needed.
Empiric Antimicrobial Selection
The selection of appropriate empiric therapy is crucial. Antibiotic regimens should be simplified if specific causative organisms are isolated.
Up to now, the standard of care for presumed aspiration pneumonia has been antimicrobial therapy with antianaerobic activity, such as clindamycin, metronidazole plus a beta-lactam antibiotic, a carbapenem, or a beta-lactam/beta-lactamase inhibitor combination agent.23,25 As clinicians’ understanding of both the pathophysiology and bacteriology associated with aspiration pneumonia grows, so too does the ability to practice effective antimicrobial stewardship by selecting optimal therapy based on individual patient circumstances. This is vital given that many of the aforementioned standard-of-care antimicrobials have, to one degree or another, been associated with Clostridioides difficile–associated disease or other adverse events, as well as increased bacterial resistance.28
The 2019 ATS/IDSA CAP diagnostic and treatment guideline update advises that the addition of anaerobic coverage to standard CAP regimens in patients in the inpatient setting is unnecessary unless a lung abscess or empyema is suspected. This is an important statement, as it suggests that a significant number of patients who would have been treated with exceptionally broad-spectrum antimicrobials, such as piperacillin/tazobactam or a carbapenem, are likely to respond as favorably to a standard CAP regimen of—for example—ceftriaxone plus a macrolide or doxycycline.2 Notably, this recommendation includes ampicillin/sulbactam (plus a macrolide or doxycycline) and moxifloxacin, both of which have inherent antianaerobic activity.
To complicate matters, patients who are most likely to develop aspiration pneumonia (e.g., stroke patients and nursing home residents) are also more likely to develop recurrent pneumonia. This leads to a greater incidence of hospital admission and more frequent receipt of IV antimicrobials, which places these patients at risk for infection owing to the presence of resistant organisms such as P aeruginosa, which require the use of broader-spectrum antimicrobials.2,12,13,16
Patients who develop aspiration pneumonia in the hospital, particularly in the ICU or after a prolonged inpatient stay, should likely be treated as HAP. Although the rate of anaerobic infections does not appear to be higher among patients who aspirate in the hospital setting, the selection of an empiric antimicrobial regimen for such patients favors drugs with antianaerobic activity, such as piperacillin/tazobactam and carbapenems.12,13,16
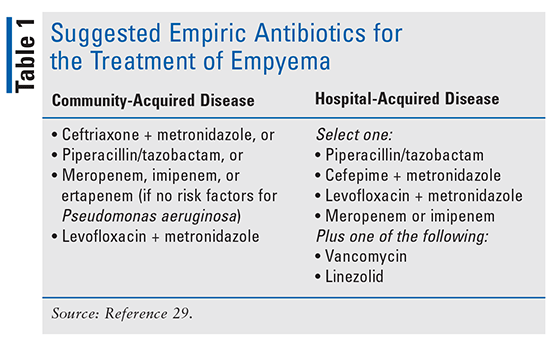
Finally, patients who have developed empyema will generally require drainage in conjunction with appropriate antimicrobial treatment in order to achieve effective resolution of disease. Once again, the location where the disease was acquired (i.e., CAP vs. HAP) will help guide antimicrobial selection, with empiric coverage of anaerobic organisms being crucial (TABLE 1). The optimal duration of antimicrobial therapy has not yet been determined, and duration will depend on response to treatment, the causative organism, illness severity, and complicating patient factors. Durations of 2 to 6 weeks or more have been required for successful disease resolution.29
Antimicrobial Prophylaxis After an Aspiration Event
It is not uncommon for prophylactic antibiotics to be initiated after an aspiration event. A survey of critical-care providers found that nearly 80% initiated prophylactic antimicrobials at the time of a macroaspiration event.30 In a study of 76 patients who received antimicrobials within the first 48 hours after macroaspiration, there was no improvement in mortality; predictably, antimicrobial use was higher in this group versus the control group, which received supportive care.31 Therefore, prophylactic antibiotics after a macroaspiration event should be avoided in favor of supportive care, as these antibiotics offer no improvement in mortality and may increase the patient’s risk of secondary infections and adverse drug-related events.
Conclusion
Antibiotic selection for treating the subset of pneumonia patients who have been diagnosed with aspiration pneumonia is not a simple matter for practitioners who are concerned with antimicrobial stewardship and with optimizing patient care while mitigating patient risk of antibiotic-related adverse events. The selection of appropriate antibiotic therapy requires an understanding of the patient’s individual risks for experiencing a macroaspiration event as well as circumstances that may prevent effective clearing of solid materials and bacteria from the airway. Furthermore, an understanding of the available antimicrobial agents and their spectrums of activity, as well as knowledge of guidelines and current literature, can inform decisions about the most effective treatment for aspiration pneumonia on a patient-by-patient basis.
REFERENCES
1. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665-671.
2. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
3. Mandell LA, Wunderink R. Pneumonia. In: Longo DL, Kasper DL, Jameson JL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw Hill Medical; 2012.
4. Dean NC, Jones BE, Jones JP, et al. Impact of an electronic clinical decision support tool for emergency department patients with pneumonia. Ann Emerg Med. 2015;66:511-520.
5. Wipf JE, Lipsky BA, Hirschmann JV, et al. Diagnosing pneumonia by physical examination: relevant or relic? Arch Intern Med. 1999;159:1082-1087.
6. Lévy M, Dromer F, Brion N, et al. Community-acquired pneumonia. Importance of initial noninvasive bacteriologic and radiographic investigations. Chest. 1988;93:43-48.
7. Ewig S, Schlochtermeier M, Göke N, Niederman MS. Applying sputum as a diagnostic tool in pneumonia: limited yield, minimal impact on treatment decisions. Chest. 2002;121:1486-1492.
8. Campbell SG, Marrie TJ, Anstey M, et al. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest. 2003;123:1142-1150.
9. van Duin D, Paterson DL. Multidrug-resistant bacteria in the community: trends and lessons learned. Infect Dis Clin North Am. 2016;30:377-390.
10. Furuya EY, Lowy FD. Antimicrobial-resistant bacteria in the community setting. Nat Rev Microbiol. 2006;4:36-45.
11. CDC. Atypical pneumonia. www.cdc.gov/pneumonia/atypical/index.html. Accessed June 4, 2021.
12. Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med. 2019;380:651-663.
13. Neill S, Dean N. Aspiration pneumonia and pneumonitis: a spectrum of infectious/noninfectious diseases affecting the lung. Curr Opin Infect Dis. 2019;32:152-157.
14. Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111:1266-1272.
15. Webb BJ, Dascomb K, Stenehjem E, Dean N. Predicting risk of drug-resistant organisms in pneumonia: moving beyond the HCAP model. Respir Med. 2015;109:1-10.
16. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
17. El-Solh AA, Pietrantoni C, Bhat A, et al. Colonization of dental plaques: a reservoir of respiratory pathogens for hospital-acquired pneumonia in institutionalized elders. Chest. 2004;126:1575-1582.
18. Hannawi Y, Hannawi B, Venkatasubba Rao CP, et al. Stroke-associated pneumonia: major advances and obstacles. Cerebrovasc Dis. 2013;35:430-443.
19. Happel KI, Odden AR, Zhang P, et al. Acute alcohol intoxication suppresses the interleukin 23 response to Klebsiella pneumoniae infection. Alcohol Clin Exp Res. 2006;30:1200-1207.
20. Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med. 2018;168:396-404.
21. Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med. 1974;56:202-207.
22. Cesar L, Gonzalez C, Calia FM. Bacteriologic flora of aspiration-induced pulmonary infections. Arch Intern Med. 1975;135:711-714.
23. Bartlett JG, Gorbach SL. Treatment of aspiration pneumonia and primary lung abscess. Penicillin G vs clindamycin. JAMA. 1975;234:935-937.
24. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68:560-566.
25. Mier L, Dreyfuss D, Darchy B, et al. Is penicillin G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intensive Care Med. 1993;19:279-284.
26. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115:178-183.
27. Wang JL, Chen KY, Fang CT, et al. Changing bacteriology of adult community-acquired lung abscess in Taiwan: Klebsiella pneumoniae versus anaerobes. Clin Infect Dis. 2005;40:915-922.
28. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127:1276-1282.
29. Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg. 2017;153:e129-e146.
30. Rebuck JA, Rasmussen JR, Olsen KM. Clinical aspiration-related practice patterns in the intensive care unit: a physician survey. Crit Care Med. 2001;29:2239-2244.
31. Dragan V, Wei Y, Elligsen M, et al. Prophylactic antimicrobial therapy for acute aspiration pneumonitis. Clin Infect Dis. 2018;67:513-518.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






