US Pharm. 2016;41(6)(Generic suppl):44-50.
ABSTRACT: Angiotensin receptor blockers (ARBs) are potential options for the treatment of high blood pressure. They are first-line agents for patients with hypertension and comorbid chronic kidney disease. Various trials have found more benefit and fewer adverse events from losartan compared to atenolol, a beta-blocker, in patients with left ventricular hypertrophy. With many of the drugs in these classes being generic, the risk of poor adherence by patients due to cost alone is minimal. Adverse effects that can be seen as a result of ARB therapy include hyperkalemia and angioedema. Based on current price estimates, the most inexpensive ARBs include irbesartan and losartan; however, choice of therapy should always be discussed and individualized.
Within the renin-angio-tensin-aldosterone system (RAAS), increases in blood pressure are achieved when angiotensin I is converted to angiotensin II by angiotensin-converting enzyme (ACE), which then binds to AT1 receptors located in various muscles and tissues such as vascular and myocardial tissue and in the liver. This conversion from angiotensin I to angiotensin II and subsequent receptor binding leads to increases in vasoconstriction, aldosterone secretion, and sympa-thetic activation. These effects ultimately lead to increased blood pressure. Angiotensin receptor blockers (ARBs) work to decrease blood pressure by preventing angiotensin II from binding to AT1 receptors. While ACE inhib-itors also block the RAAS, their effects are seen prior to the potential for AT1 receptor binding, which is why there are some differences seen in the frequency of adverse effects with each of these medication classes.1
This review will summarize the need for ARBs in the management of hypertension, evidence for their use, and the cost of available generic medications.
2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults
According to the Eighth Joint National Committee’s (JNC 8) Evidence-Based Guideline for the Management of High Blood Pressure in Adults, an ACE inhibitor or ARB should be considered first-line therapy in all patients with chronic kidney disease (CKD), as well as a thiazide-type diuretic in nonblack patients when not achieving recommended blood pressure goals.2 Within the 2014 update, evidence was presented to support the treatment of hypertensive patients aged ≥60 years to a blood pressure goal of <150/90 mmHg (Grade A recommendation), and to a blood pressure goal of <140/90 mmHg for patients aged <60 years (Grade E recommendation). There is also significant evidence to support the use of an ACE inhibitor, ARB, calcium channel blocker (CCB), or thiazide-type diuretic in the nonblack hypertensive population, including those with diabetes. FIGURE 1 illustrates the treatment algorithm for hypertensive patients as published in the JNC 8 guideline.2
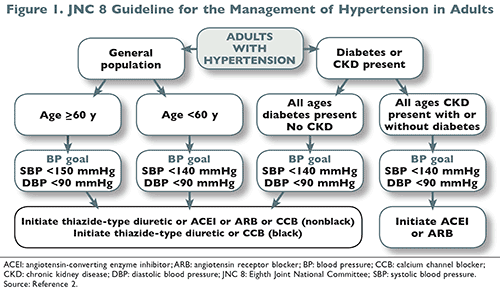
Of note, ARBs are widely prescribed due to their efficacy in lowering blood pressure and decreasing the risk of cardiovascular events and to the potential for renal protection in patients with diabetes.2 Additionally, there is evidence that losartan can help to reduce uric acid levels in patients presenting with hyperuricemia from both medication use and a diagnosis of gout.3
Major Clinical Trials of Generic ARBs
The ARB prototype, losartan, was studied extensively in the early 2000s. The LIFE trial (Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study) compared losartan with atenolol, a beta-blocker, to determine whether selective blocking of angiotensin II improves left ventricular hypertrophy (LVH) beyond reducing blood pressure over 4 years.4 It also investigated whether or not ARBs reduce cardiovascular morbidity and death. LIFE was a double-blind, randomized, parallel group trial with 9,193 participants between the ages of 55 and 80 years with diabetes, signs of LVH, and hypertension, defined by a sitting blood pressure of 160-200/95-115 mmHg. Each participant was given either losartan or atenolol once daily. Blood pressure levels in the losartan group fell more than in the atenolol group, 30.2/16.6 (SD 18.5/10.1) and 29.1/16.8 mmHg (SD 19.2/10.1), respectively. There were also fewer deaths from cardiovascular disease in the losartan group; however, these results were not statistically significant. There was a significant difference in patients having fatal or nonfatal stroke, with the results favoring losartan over atenolol (232 vs. 309; 0.75, 95% CI 0.63-0.89; P = .001).4
In regard to adverse effects, both medications were generally well tolerated; however, atenolol was found to have more bradycardia (8% vs. 1%), hypokalemia (7% vs. 4%), and albuminuria (13% vs. 7%) than losartan.4 Overall, the LIFE trial concluded that hypertensive patients with LVH benefit more from losartan than from atenolol. Losartan was also associated with fewer adverse events and less worsening of kidney function for patients with diabetes than atenolol.4
The VALUE (Valsartan Antihypertensive Long-Term Use Evaluation) trial, carried out between September 1997 and November 1999, investigated valsartan versus amlodipine for blood pressure control and events.5 It included both men and women with hypertension aged ≥50 years with high risk for cardiovascular events, defined as having ≥1 risk factors or diseases. Patients were required to have systolic blood pressure 160 to 210 mmHg and diastolic blood pressure 95 to 105 mmHg to be eligible for inclusion. Treatment began with amlodipine 5 mg daily or valsartan 80 mg daily with titration to 10 mg and 160 mg, respectively. Patients were also eligible to receive hydrochlorothiazide (HCTZ) 12.5 to 25 mg daily in order to achieve blood pressure control. The authors defined blood pressure control as <140/90 mmHg. The primary endpoint was a composite of cardiac events with stroke as a secondary endpoint. Both groups in VALUE achieved equal rates of blood pressure control (63% valsartan, 57% amlodipine). Mean blood pressure was lower at the end of the trial in the amlodipine group (by 17.3/9.9 mmHg) versus the valsartan group (by 15.2/8.2 mmHg). The rates of peripheral edema were higher in the amlodipine group (32.9%) versus the valsartan group (14.9%). No difference in cardiac endpoint was seen between the two groups; however, the incidence of myocardial infarction (a secondary outcome) was lower in the amlodipine group, which may have been related to better blood pressure control.5
Adverse Effects of ARBs
When considering starting an ARB, providers should keep in mind the adverse-effect profiles of this class of medication. In general, all ARBs will have the same potential to cause laboratory changes in potassium and other electrolytes; however, ARBs may be more likely to cause hyperkalemia than ACE inhibitors. A 2009 retrospective, observational cohort study of patients taking ACE inhibitors versus ARBs was conducted in a Veterans Affairs Medical Center looking at approximately 2,331 patients.6 After reviewing medical records over a 12-month period, the authors found that hyperkalemia (>5 mEq/L) was observed in a higher number of patients taking ARBs (31%) versus ACE inhibitors (20.4%). The number of patients with hyperkalemia still remained higher even after adjustment for renal function.6
The mechanism resulting in hyperkalemia may include causes such as decreased aldosterone production or impaired potassium secretion within the kidneys. With angiotensin II receptor blockers, the activity of aldosterone is reduced and therefore prevents the excretion of potassium from the kidneys.7 As previously discussed, ARBs are less likely to cause bradycardia than are beta-blockers and are also less likely to cause lower extremity edema, a side effect that has been reported with CCBs such as amlodipine.4,5 The rates of chronic dry cough with ARBs have been found to be similar to placebo.8 Another potential severe adverse effect is angioedema, which has been shown to occur less often in patients taking ARBs versus ACE inhibitors.9 It is important to note that ARBs are Pregnancy Category D and should be avoided in pregnancy.10
Cost Comparisons of Generic ARBs
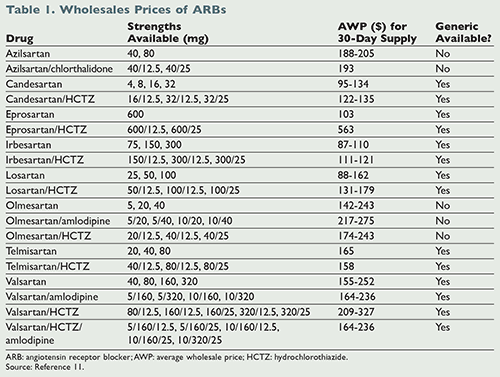
TABLE 1 summarizes the actual wholesale prices of each of the ARBs currently on the market, including combination products.11 Prices range from about $87 to over $300 for a 30-day supply. Several chain community pharmacies such as Walmart, CVS, and Walgreens offer discount plans for 30- and 90-day supplies of many generic medications. Walgreens, for example, provides a prescription savings program that offers a 30-day supply of losartan/HCTZ for $10 and a 90-day supply for $20.12 Many companies provide similar programs, and some even offer price matching. Currently, valsartan is the most widely prescribed ARB on the market, followed by losartan.13 Since both medications are generic and insurance companies frequently cover these drugs, there should be little concern with lack of adherence due to cost.
Conclusion
ARBs are recommended in varying levels of evidence within the most recent guidelines for the management of hypertension because of various mechanisms including renal protection, lowering of blood pressure, and reducing uric acid. While the evidence to support the use of ARBs versus other antihypertensives varies, ARBs have consistently shown a benefit for lowering blood pressure and have demonstrated favorable tolerability. Additionally, the generic availability of ARBs now makes it possible for many of these agents to be included on insurance formularies and to provide a wider selection when starting or adjusting therapy.
REFERENCES
1. Kirk J. Angiotensin-II receptor antagonists: their place in therapy. Am Fam Physician. 1999;59(11):3140-3148.
2. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-550.
3. Ica DA, Schoolwerth AC. Part 1. Uric acid and losartan. Curr Opin Nephrol Hypertens. 2002;11(5):475-482.
4. Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359: 995-1003.
5. Julius S, Kjeldsen SE, Weber M, et al; VALUE trial group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022-2231.
6. Sadjadi SA, McMillan JI, Jaipaul N, et al. A comparative study of the prevalence of hyperkalemia with the use of angiotensin-converting enzyme inhibitors versus angiotensin receptor blockers. Ther Clin Risk Manag. 2009;5(3):547-552.
7. Desai A. Hyperkalemia associated with inhibitors of the renin-angiotensin-aldosterone system: balancing risk and benefit. Circulation. 2008;118:1609-1611.
8. Goldberg AI, Dunlay MC, Sweet CS. Safety and tolerability of losartan potassium, an angiotensin II receptor antagonist, compared with hydrochlorothiazide, atenolol, felodipine ER, and angiotensin-converting enzyme inhibitors for the treatment of systemic hypertension. Am J Cardiol. 1995;75:793-795.
9. Johnsen SP, Jacobsen J, Monster TB, et al. Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am J Med. 2005;118:1428-1429.
10. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
11. Red Book Online [online database]. Greenwood Village, CO: Truven Health Analytics. Updated March 25, 2009. www.micromedexsolutions.com. Accessed January 26, 2016.
12. Value-priced medication list. Walgreens Prescription Savings Club. March 2012. www.walgreens.com/images/psc/pdf/PSC_2_0_VPG_List_FINAL_32312.pdf. Accessed March 1, 2016.
13. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
To comment on this article, contact rdavidson@uspharmacist.com.






