US Pharm. 2023;48(6):HS2-HS7.
ABSTRACT: Prostate cancer, the most frequent cancer among men after skin cancer, is the second leading cause of cancer-related deaths in men. Typically, localized prostate cancer is asymptomatic; however, prostate cancer is more commonly diagnosed after the onset of symptoms such as urinary hesitancy, retention, dysuria, and erectile dysfunction. Treatment often involves a combination of observation, surgery, hormonal therapy, corticosteroids, and chemotherapy including immunotherapy and targeted therapy. Pharmacists are vital to the care of patients with prostate cancer in the areas of medication access, counseling and education, and the identification and management of life-threatening adverse effects of agents for prostate cancer.
Prostate cancer, the most frequent cancer among men after skin cancer, is the second leading cause of cancer-related deaths in men.1,2 The incidence of prostate cancer has increased by 3% each year since 2014.2,3 It is estimated that 288,300 new cases of prostate cancer will be reported in the United States in 2023, with 34,700 cases resulting in death.2,3 Over a life span, approximately one in eight men will be diagnosed with prostate cancer.2 Presumed risk factors are age greater than 40 years, African American race, and genetic factors such as germline mutations and inherited polymorphisms that lead to increased androgen receptor activation.2,4 Typically, localized prostate cancer is asymptomatic; however, prostate cancer is more commonly diagnosed after the onset of symptoms, including urinary hesitancy, retention, dysuria, hematuria, and erectile dysfunction. Ureteral dysfunction, frequency, hesitancy, dribbling, and impotence are symptoms of locally invasive disease.5
Prostate-specific antigen (PSA) screening is a tool to assess the amount of PSA protein in the blood.5 A higher PSA number is typically associated with a greater chance of prostate cancer. Physical activity and diet are key components in prevention because certain risks pertaining to weight and diet may increase the likelihood of diagnosis.
The most widely used staging method for prostate cancer is the American Joint Committee on Cancer’s TNM system, which is based on five key aspects: the extent of the main tumor (T), whether the cancer has spread to nearby lymph nodes (N), whether the cancer has metastasized (M), PSA level at diagnosis, and Gleason score (determined by the prostate biopsy results).6-8 The Gleason score, a measure of how likely cancer is to grow and spread quickly, grades tumors on a scale from 1 to 5; a grade 1 tumor looks like normal prostate tissue, and a grade 5 tumor looks highly abnormal. The Gleason scores of the two most predominant tumor areas are then added to determine the Gleason sum (e.g., 3 + 4 = 7).8 Prostate cancers are typically divided into three groups: 1) a cancer with a Gleason score of 6 or less is well differentiated or low-grade; 2) a Gleason score of 7 means moderately differentiated or intermediate-grade; and 3) a Gleason score of 8 to 10 signifies poorly differentiated or high-grade.8
Treatment
The treatment of prostate cancer frequently involves a combination of observation, surgery, hormonal therapy, corticosteroids, and chemotherapy including immunotherapy and targeted therapy. In some patient populations, treatment is not recommended; instead, active surveillance (wherein a PSA blood test is conducted every 6 months and a digital rectal examination is performed at least once per year) or observation (often when life expectancy is <10 years) may be employed.6
Nonpharmacologic treatments include orchiectomy, prostatectomy, and transurethral resection of the prostate.9 Prostatectomy, which involves removal of the prostate, may be done either via open surgery or laparoscopically.6 Adverse effects (AEs) of prostatectomy may include urinary incontinence and erectile dysfunction; the risk is increased by anastomotic strictures.8
Hormonal Therapy
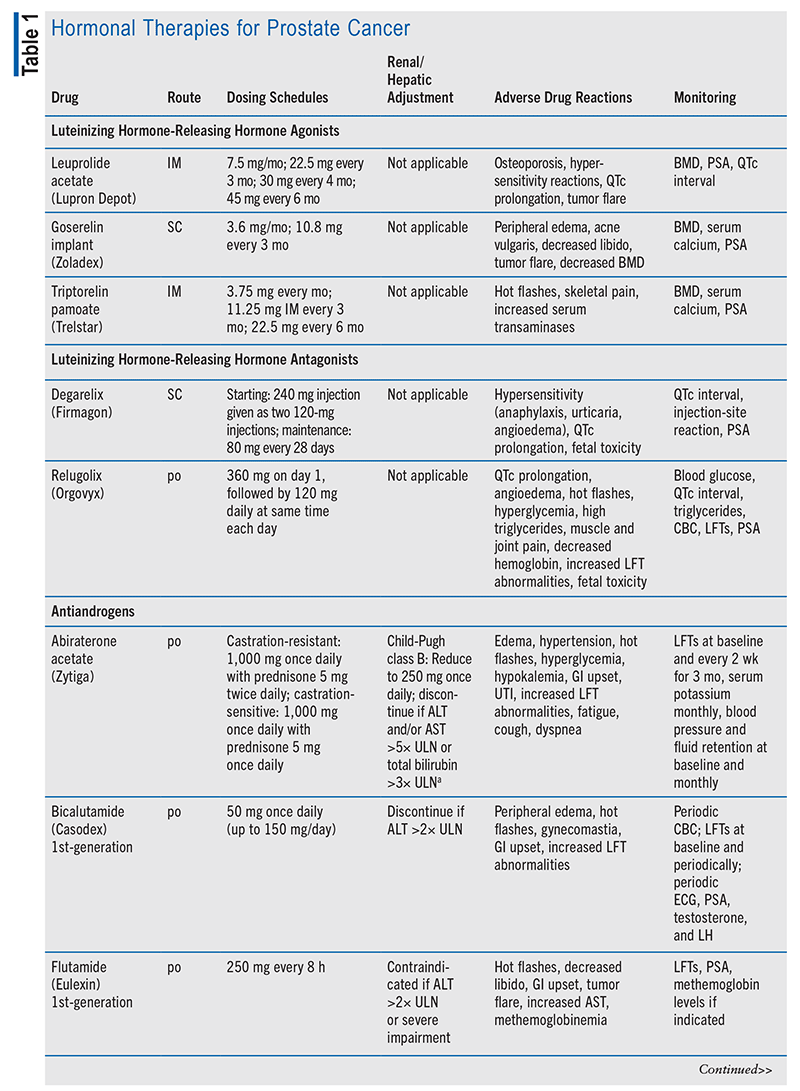
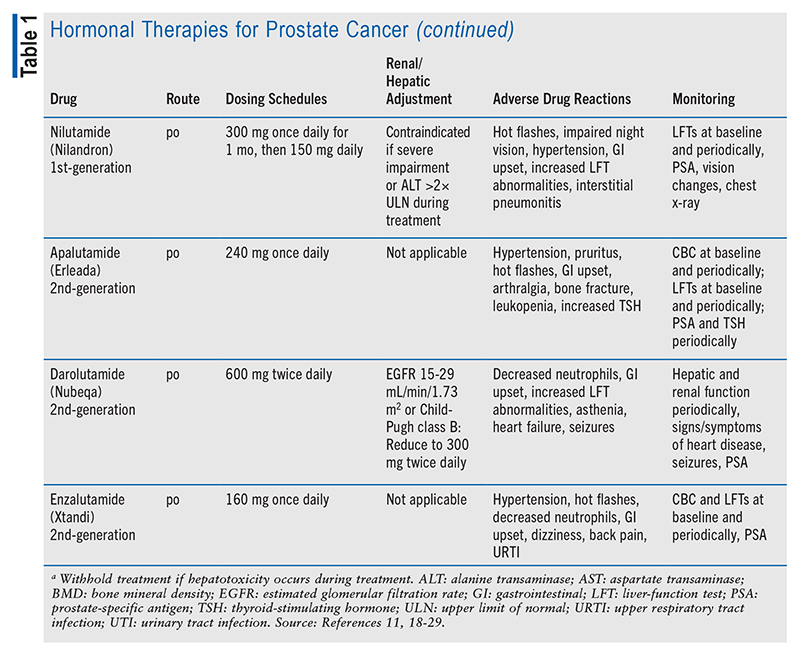
The aim of hormonal therapy is to prevent prostate cancer cell proliferation by lowering the level of testosterone to castration levels.6 Androgen deprivation therapy (ADT) is achieved through orchiectomy or the use of either a luteinizing hormone-releasing hormone (LHRH) agonist with an antiandrogen or an LHRH antagonist administered alone.10 If an LHRH agonist is given before an antiandrogen, a tumor flare may occur, causing AEs such as bone pain and difficulty urinating, but these effects can be avoided if the patient is given an antiandrogen for a few weeks prior to starting the LHRH agonist. LHRH antagonists do not cause a tumor flare.6
In advanced disease, it is insufficient to block androgen formation in the testicles alone; other tissues that make androgens require suppressive therapy as well. The antiandrogen abiraterone prevents the formation of testosterone in the adrenal glands by blocking CYP17, making it necessary for the patient to continue ADT and add on a corticosteroid to avoid symptoms of adrenal suppression.6,9,11 Both castration-sensitive prostate cancer and hormone-sensitive prostate cancer (i.e., the cancer is responsive to low testosterone level) are controlled with these testosterone-lowering medications or surgery, but both castration-resistant prostate cancer and hormone-refractory prostate cancer (i.e., the cancer continues to grow in the presence of low testosterone level) need further treatment.6 In nonmetastatic castration-resistant disease, the patient may continue ADT with an antiandrogen (apalutamide, darolutamide, or enzalutamide).9 Refer to TABLE 1 for a summary of hormonal therapy medication options.
Chemotherapy
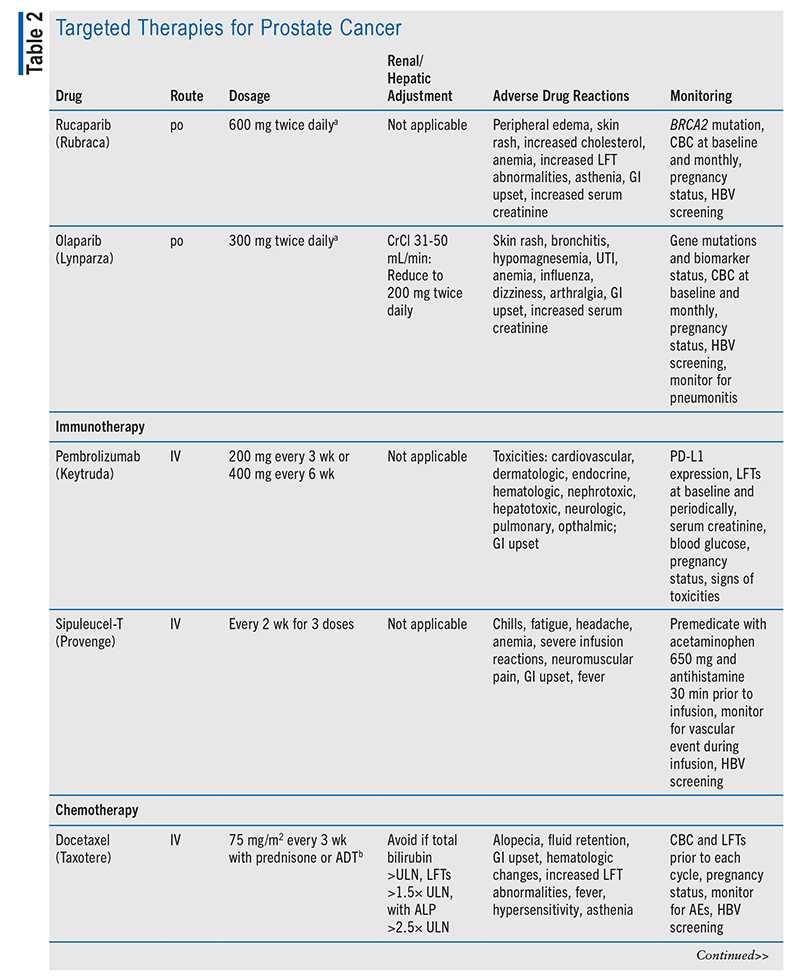
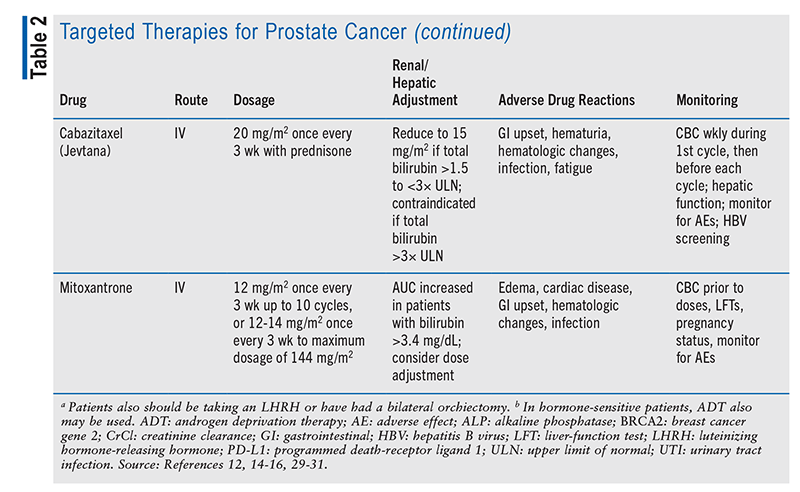
Chemotherapy is used when the prostate cancer has metastasized. Chemotherapy agents are given one at a time and usually include docetaxel, cabazitaxel, mitoxantrone, or estramustine.6 For example, in castration-naïve metastatic prostate cancer (i.e., patient not on ADT at time of diagnosis), ADT plus docetaxel, a taxane (i.e., microtubule inhibitor that prevents cellular replication), may be used.9 Upon docetaxel failure, cabazitaxel (a microtubule inhibitor similar to docetaxel) may be considered; or, if other methods are not tolerated, the topoisomerase II inhibitor mitoxantrone may be considered. In metastatic and castration-resistant cases, ADT should continue, along with docetaxel plus prednisone, an antiandrogen, or sipuleucel-T (an immunotherapy)—with the agent selected being one that the patient has not previously failed.9
Immunotherapy
Sipuleucel-T, a cancer vaccine that boosts the immune system to help attack cancer cells, is usually reserved for prostate cancer that no longer responds to hormonal therapy but is not causing major AEs.6,12 This vaccine is patient-specific, meaning that it is created by sending the patient’s WBCs to a laboratory, where it is mixed with prostatic acid phosphatase (a protein from prostate cancer cells), and then administering it back to the patient. This process is repeated for a total of three doses. This therapy can help the patient live longer but will not stop the growth of prostate cancer.12,13
Immune checkpoint inhibitors are used in patients whose tumor expresses programmed death receptor 1 (PD-1; a checkpoint protein on T cells) and are often employed for metastatic disease.6 Pembrolizumab is a PD-1 inhibitor recommended for use in metastatic castration-resistant prostate cancer that has not adequately responded to docetaxel or hormonal therapy.9,14 Pembrolizumab boosts the body’s immune response against prostate cancer cells; however, sometimes the immune system attacks other cells, causing life-threatening AEs such as pneumonitis, myocarditis, and hyperglycemia-related diabetic ketoacidosis, so it is imperative that the medical team identify and treat these AEs quickly and appropriately.9
Targeted Therapy
In castration-resistant cases, genetic testing must be completed to determine whether targeted therapy could be used. Poly (ADP)-ribose polymerase (PARP) enzymes normally help repair DNA inside cells after damage. In cells with BRCA1 and BRCA2 (breast cancer genes 1 and 2) mutations, a PARP inhibitor makes it difficult for the cell to repair damaged DNA and cause cell death.6 Olaparib and rucaparib are PARP inhibitors whose activity involves suppressing DNA repair mechanisms, leading to cell death; these agents may be used in patients expressing BRCA1 or BRCA2 mutations.9,15-17 Refer to TABLE 2 for a summary of targeted therapy medication options.
The Pharmacist’s Role
Pharmacists are essential in the management and treatment of prostate cancer. One role is to provide medication counseling and education to the patient and family members. Informing patients about their treatment regimen, as well as how the medication works and what side effects to expect, is important. Many of the newer medications, including immunotherapy and targeted therapies, have AEs that patients must monitor for at home and understand when to report.
The pharmacist also plays a part in managing any medication interactions between the treatment regimen and any at-home medications the patient is taking. As part of the medical team, the pharmacist must assess the treatment regimen and ensure that a patient taking abiraterone—which depletes the adrenal production of corticosteroids—is given stress-dose corticosteroids in the hospital even if the abiraterone has been discontinued.
Chemotherapy regimens are another avenue for pharmacist involvement. As noted in TABLE 2, chemotherapeutic agents require dosing adjustments based on various laboratory parameters. This is an excellent opportunity for pharmacists to monitor therapy and dosing throughout treatment.
Another area pharmacists may be involved in includes specialty pharmacy and the management of PARP inhibitors and immunotherapy. Monitoring AEs and CBC results is important to treatment success. In immunotherapy, AEs may quickly become life-threatening. The National Comprehensive Cancer Network has specific guidelines for treating immune-related adverse events (IRAEs), often beginning with stopping the agent and giving sufficient corticosteroids to halt the immune system’s response to the offending agent.9 Quick diagnosis of IRAEs saves lives, and frequently the medical team may not know how to treat the patient if a correct medication history is not performed.6
Pharmacists play a vital part in medication adherence, as many of these agents are expensive and require patient assistance or grant funding to ensure that the patient receives them on an ongoing basis. Communicating with patients about their regimen, AEs, and management postoperatively could lead to better adherence to medications and improved clinical outcomes.
Prostate cancer confers an increased risk of secondary cancers, including small-intestine cancer, soft-tissue cancer, bladder cancer, rectal cancer, and acute myeloid leukemia.6 Pharmacists should therefore educate patients on their increased risk and important screening options. Prostate cancer may be metastatic, most often affecting the bones first.6 Pharmacists are involved in managing these therapy regimens as well as mitigating AEs. When providing care beyond the treatment stage, including prevention, screening, and posttreatment education, the pharmacist plays a key role in patient care.
REFERENCES
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
2. American Cancer Society. Key statistics for prostate cancer. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed April 1, 2023.
3. American Cancer Society. Cancer Facts & Figures 2023. Atlanta, GA: American Cancer Society; 2023.
4. Hsieh K, Albertsen PC. Populations at high risk for prostate cancer. Urol Clin North Am. 2003;30(4):669-676.
5. Crona DJ, Cipriani AB. Prostate cancer. In: DiPiro JT, Yee GC, Haines ST, et al, eds. DiPiro’s Pharmacotherapy: A Pathophysiologic Approach. 12th ed. New York, NY: McGraw-Hill Medical; 2023.
6. American Cancer Society. Treating prostate cancer. www.cancer.org/cancer/prostate-cancer/treating.html. Accessed March 9, 2023.
7. Iczkowski KA. Current prostate biopsy interpretation: criteria for cancer, atypical small acinar proliferation, high-grade prostatic intraepithelial neoplasia, and use of immunostains. Arch Pathol Lab Med. 2006;130(6):835-843.
8. American Cancer Society. Early detection, diagnosis, and staging. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging.html. Accessed March 23, 2023.
9. Schaeffer EM, Srinivas S, Adra N, et al. NCCN Guidelines® insights: prostate cancer, version 1.2023. J Natl Compr Canc Netw. 2022;20(12):1288-1298.
10. Walsh PC. Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. J Urol. 1997;158(4):1623-1624.
11. Zytiga (abiraterone acetate) product information. Horsham, PA: Janssen Biotech, Inc; August 2021.
12. Provenge (sipuleucel-T) product information. Seal Beach, CA: Dendreon Pharmaceuticals LLC; July 2017.
13. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422.
14. Keytruda (pembrolizumab) product information. Whitehouse Station, NJ: Merck & Co, Inc; August 2021.
15. Lynparza (olaparib) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; October 2022.
16. Rubraca (rucaparib) product information. Boulder, CO: Clovis Oncology, Inc; December 2022.
17. Cleary JM, Aguirre AJ, Shapiro GI, D’Andrea AD. Biomarker-guided development of DNA repair inhibitors. Mol Cell. 2020;78(6):1070-1085.
18. Lupron Depot (leuprolide acetate) product information. North Chicago, IL: AbbVie Inc; April 2022.
19. Zoladex (goserelin implant) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2012.
20. Trelstar (triptorelin pamoate) product information. Ewing, NJ: Verity Pharmaceuticals, Inc; March 2023.
21. Firmagon (degarelix) product information. Parsippany, NJ: Ferring Pharmaceuticals Inc; February 2020.
22. Orgovyx (relugolix) product information. Brisbane, CA: Myovant Sciences, Inc; March 2023.
23. Casodex (bicalutamide) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; December 2008.
24. Eulexin (flutamide) product information. Kenilworth, NJ: Schering Corporation; 2000.
25. Nilandron (nilutamide) product information. St. Michael, Barbados: Concordia Pharmaceuticals, Inc; 2015.
26. Erleada (apalutamide) product information. Horsham, PA: Janssen Products, LP; February 2018.
27. Nubeqa (darolutamide) product information. Whippany, NJ: Bayer Healthcare Pharmaceuticals Inc; July 2019.
28. Xtandi (enzalutamide) product information. Northbrook, IL: Astellas Pharma US, Inc; September 2022.
29. Taxotere (docetaxel) product information. Bridgewater, NJ: sanofi-aventis U.S. LLC; November 2021.
30. Jevtana (cabazitaxel) product information. Bridgewater, NJ: sanofi-aventis U.S. LLC; February 2021.
31. Mitoxantrone product information. Lake Forest, IL: Hospira Inc; April 2021.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






