US Pharm. 2023;48(5):HS2-HS6.
ABSTRACT: Opioid use disorder (OUD) is a chronic disorder with potentially serious health outcomes, including disability, relapse, overdose, and death. Suboxone, a fixed-dose combination of buprenorphine and naloxone, relieves cravings to use and withdrawal symptoms and substantially reduces OUD-related mortality. Appropriately managing acute, severe pain in OUD patients prescribed Suboxone, especially with opioid-based pharmacotherapy, may be challenging due to the potential for high tolerance levels and fatal side effects. Pharmacists should work with the healthcare team to develop and optimize patient-centered care plans that incorporate nonpharmacologic approaches, nonopioid analgesia, combination nonopioid/opioid analgesia at calibrated doses, and steps to prevent adverse side effects.
Opioid use disorder (OUD) poses serious social and health problems in communities across the United States. Of the 107,622 drug overdose deaths in 2021, approximately 75% of them involved opioids.1 These statistics reflect a disturbing trend because they are approximately 15% greater than 2020 numbers.1 From 2014 to 2017, death from opioid overdose was linked to decreased life expectancy.2 The modern-day “opioid crisis” traces its origins to the mid-1990s and the explosion of prescription opioids, such as hydrocodone and oxycodone.3 This initial crisis was followed by the increased use of heroin, a cheaper and more potent alternative to prescription opioids.3 The latest culprit is fentanyl, which is 50 times more potent than heroin and 100 times more potent than morphine.4 Fentanyl and other synthetic opioids now account for more than 80% of opioid overdose deaths.5
Medication-assisted treatment (MAT), a mainstay in the management of OUD, significantly reduces the risk of death due to overdose.6 Currently, there are three FDA-approved medications: naltrexone, an opioid receptor antagonist; methadone, an opioid receptor full agonist; and buprenorphine, an opioid receptor partial agonist (see TABLE 1).7 Opioid agonist treatment, such as that achieved with buprenorphine and methadone, is generally preferred in the treatment of moderate-to-severe OUD.6 Whereas a patient must fully complete detoxification before beginning treatment with naltrexone, methadone and buprenorphine treatment can be started immediately—yet cautiously—to avoid precipitating withdrawal.8 Although all three medications display similar efficacy in treating OUD, overdose and death following relapse are worse with naltrexone compared with opioid receptor agonists.9-11 This is partly due to the fact that there is loss of opioid tolerance with naltrexone but not with methadone or buprenorphine.10
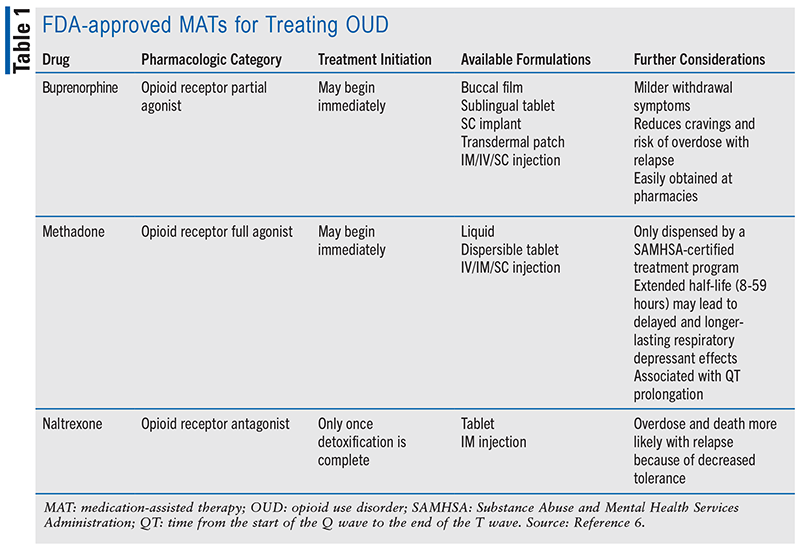
Buprenorphine has emerged as the agent of choice to treat OUD. From 2004 to 2011, its use increased by a staggering 2,318% (vs. a 37% increase with methadone), which contributed to a concomitant rise in its abuse.12-14 Buprenorphine binds to all three opioid receptors: mu opioid receptor (MOR), kappa opioid receptor (KOR), and delta opioid receptor (DOR).3,15 It acts as an antagonist at both the KOR (to elicit antihyperalgesia) and DOR.16 Contention remains whether it acts at the MOR as a full agonist, partial agonist, or biased agonist, but most literature describes it as a partial agonist.15,17 Regardless, it binds to the MOR with high affinity, dissociates slowly, and displays low intrinsic activity (i.e., relative ability of a drug-receptor complex to produce a maximum functional response), which results in decreased pain and increased reward-related behavior.18-20 MOR occupancy by buprenorphine for a long duration leads to milder withdrawal symptoms and reduces craving and risk of overdose following heroin or fentanyl use.18 This diverse pharmacology garners buprenorphine a better side-effect profile than other opioid receptor agonists.21
The most prescribed variation of buprenorphine for treating OUD is Suboxone, a 4:1 formulation of buprenorphine and naloxone (an opioid receptor antagonist used as an opioid overdose antidote). It is a highly successful treatment that can be physician-prescribed and easily obtained at pharmacies. This is in stark contrast to methadone, which must be prescribed and administered by a licensed facility. One major challenge to MAT in OUD patients may be encountered when these same individuals must be managed for acute, severe pain. Achieving appropriate pain control, especially through opioid-based pharmacotherapy, may be difficult due to high tolerance levels and potentially fatal side effects. This article will discuss patient-centered care plan options and the role of the pharmacist in identifying viable treatment regimens that reconcile the two opposing components of maintaining OUD treatment and effectively treating acute, severe pain.
CDC Guidelines on Opioid-Based Analgesia
CDC clinical practice guidelines focus on the treatment of acute and chronic pain in patients aged 18 years and older.22 These guidelines include recommendations for special populations, such as patients who are elderly, pregnant, or have a history of substance abuse disorder, but they exclude cancer-related pain, sickle cell disease, palliative care, and end-of-life care.22 The guidelines were recently updated in 2022 using data from five systematic reviews and address four areas: the decision to initiate opioids; opioid selection and dosing; duration and monitoring of therapy; and addressing the risks of misuse or abuse.22 Nonopioid therapies are preferred for treating acute, mild pain since they are as efficacious as opioids for musculoskeletal pain, dental pain, mild postoperative pain, kidney stones, and headaches (including migraines) and are associated with fewer side effects.22 Opioids are recommended first-line for moderate-to-severe pain (e.g., burns, trauma) or when nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated (e.g., preexisting renal disease, congestive heart failure, cirrhosis) and should be prescribed as immediate-release formulations as needed (vs. scheduled).22
Opioids should not be initiated without a plan for discontinuation. In an opioid-naïve patient, the lowest effective dose is approximately 20 to 30 morphine milligram equivalents (MMEs)/day (approx. 5 to 10 MMEs/dose).22 Dosages greater than 50 MMEs/day are unlikely to further reduce pain and carry greater risks of misuse, side effects, overdose, and death.22 If administered at a high dose, naloxone and overdose education should be provided, and follow-up should occur frequently. Initial follow-up is recommended 1 to 4 weeks after starting therapy to assess safety and efficacy and dose adjust if necessary.22 Patients already taking an opioid may require additional therapy to control their pain severity. If additional opioids are used, a taper down to baseline should be implemented as soon as possible to avoid adverse withdrawal.22
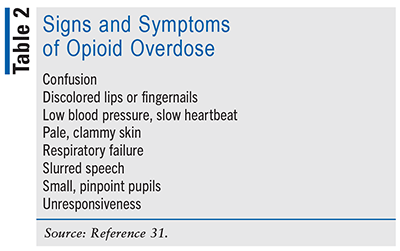
Patients who use opioids for more than 90 days after initiation of therapy have a significantly increased risk of developing new OUD.22 Therefore, nonopioid therapies are also preferred to treat subacute and chronic pain. In the setting of fibromyalgia or headache, for example, the risks of opioid use outweigh the benefits.22 Opioids should not be discontinued abruptly, except when there are signs of overdose (see TABLE 2).22 Patients with long-term and/or high-dose opioid use may require a tapering-off period lasting several months to even years. It is important to monitor for withdrawal symptoms (e.g., mydriasis, anxiety, tremor, agitation) because they could serve as barriers to tapering off and lead to dependence. The decision to taper off should be patient-centric, as patients who are self-motivated are more likely to be successful.22
Managing Acute Pain in Patients With OUD
The most effective patient care involves creating a care plan that prioritizes an individual’s specific health needs and desired outcomes. Patient-centered care lends itself to the patient playing an active role in the decision-making process. Patient involvement and collaboration with a multidisciplinary team allows for the development of a comprehensive and integrative treatment plan that encompasses multifaceted approaches across disciplines. Integration of the patient into the healthcare team is linked to improved overall health outcomes.
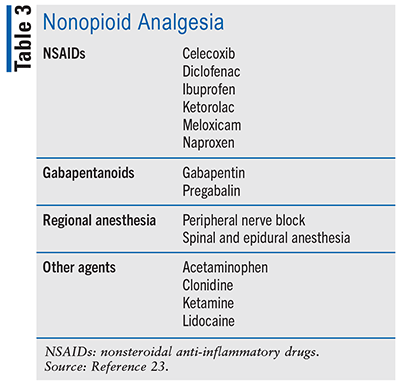
Individuals struggling with opioid dependence and undergoing active treatment may find opioid-based pharmacotherapy daunting. They may even be unwilling to take an opioid to manage their pain. Providers should be willing to discuss both nonopioid analgesic strategies (see TABLE 3) and nonpharmacologic approaches (e.g., hypnosis, mindfulness, acupuncture, manipulations, yoga, massage, heat and cold therapy, biofeedback, transcutaneous electrical nerve stimulation) to alleviate pain.23,24 These options should be maximized before adding opioid therapy and remain as adjuncts if opioid therapy is initiated. This approach helps to mitigate pain while not reintroducing the offending opioid. It also minimizes the risk of overdose associated with additional opioid analgesia. Similar to opioids, nonopioid agents have warnings and contraindications about which the patient should be advised. Of note, many alternate medications can be used as sole analgesia only in the setting of mild-to-moderate pain.
When managing acute, severe pain, it is reasonable to consider opioid analgesics in combination with acetaminophen or NSAIDs. Buprenorphine is ideal because it displays no analgesic ceiling effect.23 Given that OUD patients may have a high opioid tolerance due to previous and current opioid use, the team may experience some difficulty achieving adequate pain control at normal empiric doses and should, therefore, consider administering higher doses of full MOR agonists in shorter intervals. Although there is no consensus about what the most appropriate care plan is, current evidence suggests remaining on the current dosage of buprenorphine and supplementing as needed. Depending on the pain severity and current dosing, another recommendation to achieve pain control could be to increase the buprenorphine dose up to 32 mg/day in divided doses to avoid introducing other medications.23
Adding other opioids to a buprenorphine regimen poses several challenges, including high opioid tolerance, subtherapeutic treatment of pain, and increased risk of opioid overdose. However, tapering and discontinuing buprenorphine is associated with inferior outcomes and a higher risk of return to illicit opioid use. Fear of subtherapeutic pain management stems from evidence that buprenorphine and MOR full agonists compete at MORs. Discontinuing buprenorphine may require higher dosages of alternate opioids, imparting a higher risk of overdose.23 Another strategy is to decrease the buprenorphine dose and supplement with shorter-acting opioids to decrease the amount of drug at MORs and the overall risk associated with overlap. Given the shorter analgesic half-life of buprenorphine, the total daily dose could also be divided by three and administered every 8 hours for better analgesic effect.25 Yet another strategy is to convert the patient to methadone, which has less binding affinity for MORs, allowing a full MOR agonist to bind and exert more robust analgesia.26 Mixed agonists and antagonists such as pentazocine, nalbuphine, and butorphanol should be avoided due to potential displacement at receptors, precipitating an acute withdrawal.23
It is best to stay ahead of the pain by scheduling pharmacologic agents rather than dosing as needed since treating pain only when it flares may induce more anxiety and tension in the patient. It is also essential to employ proper patient-specific discontinuation of any opioid following resolution of the acute pain episode. Although pain management is often challenging, suboptimal control may lead to negative consequences and fuel addiction; therefore, the patient’s pain level should be continuously monitored.
Challenges to Care
Challenges encountered when treating acute, severe pain in OUD patients receiving Suboxone are multifaceted. Primary issues include patient fear of relapse, missed opportunities to educate providers, and insufficient patient support and counseling. Adequately treating pain is an essential component of quality healthcare; however, inadequate treatment is common.26 The growing number of OUD patients (likely undergoing MAT) requires pharmacists and other healthcare team members to rethink treatment options, which is complicated by the fact that the underlying mechanisms of pain and addiction are not fully understood.27
Persistent pain may encourage OUD patients to seek relief through self-medicating, thereby increasing the chance for relapse.27 Ten percent of surgical patients continue to use opioids for 1 year postdischarge despite expectations that postoperative pain will subside within the first month following surgery.28 Since a personal history of substance use disorder is the strongest predictor of future opioid misuse, individuals in recovery are at highest risk for postoperative opioid abuse.28 Individuals with a history of opioid dependence are more likely to feel apprehensive when undergoing surgery because of the possibility of relapse coupled with valid fears about undertreated pain.
Physicians may reserve prescribing opioids across all patient populations because of fears of cognitive, respiratory, and psychomotor side effects; iatrogenic drug addiction; and prescription drug diversion.26 This reluctance may be greater with patients with a documented history of OUD.26 Prescribers may also withhold or undertreat with opioids out of concern about being manipulated by drug-seeking individuals.26 Pain is subjective, so assessing its presence and severity may be exacting. Reports of acute pain with objective findings are less likely to be manipulative gestures than reports of chronic pain with vague presentations. Using clinical tools that assess objective evidence of pain mitigates unethical behavior by drug-seeking individuals and supports opioid-based analgesia in patients with a history of dependence. Patients dependent on opioids and being treated for acute pain may be perceived as demanding because of their distrust of the medical community, concerns about being stigmatized, and/or fears that their pain will be undertreated or that their opioid treatment may be altered or discontinued altogether.26 Well-founded patient anxiety related to these concerns can be profound and may complicate attaining adequate pain relief.26
Pain control can likely be achieved in individuals with a history of opioid dependence if healthcare providers follow CDC guidelines as well as those put forward by the Joint Commission on Accreditation of Healthcare Organizations (standards for pain management) and the World Health Organization (stepladder approach to pain treatment).29 Legal concerns about using opioid pain medications in OUD patients can be mitigated by clear documentation of indication, dose, dosing interval, and amount provided.29 Acute pain is treated in a similar fashion for all patients, regardless of medical history; follow-up is important to prevent relapse. To reduce the potential for relapse or abuse, it is important that only one physician provide all pain medication prescriptions and reduce the opioid dose to a minimum effective dose; be aware of tolerance potential; wean periodically to reassess pain control; and use nonpsychotropic pain medications when possible.29 Controlling medication access also decreases the chance for relapse.29 This may be achieved by giving the medication at fixed intervals and arranging for its distribution by someone other than the patient. Fixed-interval dispensing may also minimize conflict between patients and caregivers.29 Lack of uniform care provided to OUD patients may stem from insufficient and/or conflicting clinical data that have yielded underdeveloped algorithms.30 Limited access to addiction counseling services, a valuable tool for supporting OUD patients undergoing treatment, is another barrier to care in this patient population.30
Role of the Pharmacist
Pharmacists serve as the medication experts on a healthcare team. In situations as challenging as managing acute, severe pain in the face of MAT, it is vital that the provider and other members of the healthcare team be fully involved in the plan and aware of the expected outcomes. Pharmacists can communicate and educate on Suboxone components, opioid overdose, and reversal agents that could be prioritized in the care plan. When proposing the incorporation of Suboxone into a pain regimen, concern may lie with the naloxone component blunting opioid effects. It should be discussed that there is not a significant amount of naloxone absorbed via the oral and transmucosal route; rather, it is utilized in this formulation to reduce the risk of diversion with IV use.23 In circumstances when buprenorphine is continued, verification of current regimen by the dispensing pharmacy should be done to guarantee continuation of proper dosing. Pharmacists can also have an impact by recognizing when formulated care plans place patients at increased risk for overdose. Education and recognition of the potential overdose risk associated with opioid analgesics are critical (see TABLE 2).31
Pharmacists should stress the importance of frequent monitoring and ensure that patients have an active naloxone order while inpatient as well as outpatient if appropriate. The CDC reports that “only one naloxone prescription is dispensed for every 70 high-dose opioid prescriptions.”32 Pharmacist-led counseling on why the product is important and its proper use should be provided to patients and close acquaintances. Due to known risks for opioid-induced constipation, pharmacists may also recommend monitoring bowel movements. When necessary, osmotic and stimulant laxatives could be prescribed for as-needed use and monitored to determine whether escalation of treatment is warranted. Upon resolution of the pain, proper measurements for tapering opioid analgesics should be implemented to prevent adverse withdrawals. Finally, addressing the stigma of opioid dependence is important when optimizing care. Public perception is that individuals addicted to prescription opioids lack self-discipline and are themselves to blame for the problem. When discussing treatment plans, it is important to avoid language that casts addiction in a negative light. Terminology such as “druggy,” “drug abuser,” “addict,” and “junkie” can create distrust and hinder care. Using “person-first” language that focuses on the individual rather than his/her condition is essential for stigma reduction. More appropriate terminology conveys that the individual is “a person with a substance use disorder.”33 Collectively, this approach fosters mutual trust between a patient and the healthcare team, resulting in higher satisfaction with pain control and care overall.
REFERENCES
1. CDC. U.S. overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. May 11, 2022. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm. Accessed March 1, 2023.2. Kochanek KD, Anderson RN, Arias E. Changes in life expectancy at birth, 2010-2018. National Center for Health Statistics, Health E-Stats. www.cdc.gov/nchs/data/hestat/life-expectancy/lifeexpectancy-H.pdf. Accessed March 1, 2023.
3. Sivils A, Lyell P, Wang JQ, et al. Suboxone: history, controversy, and open questions. Front Psychiatry. 2022;13:1046648.4. Wilson N, Kariisa M, Seth P, et al. Drug and opioid-involved overdose deaths—United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297.
5. CDC. Death rate maps & graphs. June 2, 2022. www.cdc.gov/drugoverdose/deaths/index.html. Accessed March 1, 2023.6. Substance Abuse and Mental Health Services Administration. TIP 63: Medications for opioid use disorder. 2021. https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf. Accessed March 1, 2023.
7. Ghanem N, Dromgoole D, Hussein A, Jermyn RT. Review of medication-assisted treatment for opioid use disorder. J Osteopath Med. 2022;122:367-374.8. Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23:63-75.
9. Ma J, Bao YP, Wang RJ. Effects of medication-assisted treatment on mortality among opioids users: systematic review and meta-analysis. Mol Psychiatry. 2019;24:1868-1883.10. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375:357-368.
11. Degenhardt L, Larney S, Kimber J, et al. Excess mortality among opioid-using patients treated with oral naltrexone in Australia. Drug Alcohol Rev. 2015;34:90-96.12. Schiller EY, Goyal A, Mechanic OJ. Opioid overdose. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023-.
13. Crane EH. Emergency department visits involving buprenorphine. In: The CBHSQ Report. Rockville, MD: Substance Abuse and Mental Health Services Administration (US); 2013-.14. Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network (DAWN): findings from drug-related emergency department visits, 2021. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration.
15. Pergolizzi J, Aloisi A, Dahan A, et al. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract. 2010;10(5):428-450.16. Infantino R, Mattia C, Locarini P, et al. Buprenorphine: far beyond the “ceiling”. Biomolecules. 2021:11(6):816.
17. Raffa RB, Ding Z. Examination of the preclinical antinociceptive efficacy of buprenorphine and its designation as full- or partial-agonist. Acute Pain. 2007;9(3):145-152.18. Walton MR, Bruce RD. Clinical pearls for buprenorphine treatment. Prim Health Care. 2021;11(1):362.
19. Darcq E, Kieffer BL. Opioid receptors: drivers to addiction? Nat Rev Neurosci. 2018;19:499-514.20. Feng Y, He X, Yang Y, et al. Current research on opioid receptor function. Curr Drug Targets. 2012;13(2):230-246.
21. Khanna IK, Pillarisetti S. Buprenorphine—an attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res. 2015;8:859-870.22. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95.
23. Carr D. Management of acute pain in adults with opioid use disorder. www.uptodate.com/contents/management-of-acute-pain-in-adults-with-opioid-use-disorder. Accessed March 1, 2023.24. Canadian Cancer Society. Complementary therapies for pain. www.cancer.ca/en/treatments/side-effects/pain/complementary-therapies-for-pain. Accessed March 1, 2023.
25. Buresh M, Ratner J, Zgierska, A, et al. Treating perioperative and acute pain in patients on buprenorphine: narrative literature review and practice recommendations. J Gen Intern Med. 2020;35:3635-3643.26. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144:127-134.
27. Wachholtz A, Foster S, Cheatle M. Psychophysiology of pain and opioid use: implications for managing pain in patients with an opioid use disorder. Drug Alcohol Depend. 2015;146:1-6.28. Myers J, Compton P. Addressing the potential for perioperative relapse in those recovering from opioid use disorder. Pain Med. 2018;19:1908-1915.
29. Prater CD, Zylstra RG, Miller KE. Successful pain management for the recovering addicted patient. Prim Care Companion J Clin Psychiatry. 2002;4:125-131.30. Callister C, Lockhart S, Holtrop JS, et al. Experiences with an addiction consultation service on care provided to hospitalized patients with opioid use disorder: a qualitative study of hospitalists, nurses, pharmacists, and social workers. Subst Abus. 2022;43:615-622.
31. Indian Health Service. Opioid overdose. www.ihs.gov/opioids/naloxone/opioidoverdose. Accessed March 1, 2023.32. CDC. Naloxone. February 8, 2022. www.cdc.gov/opioids/naloxone/index.html. Accessed March 1, 2023.
33. Johns Hopkins Medicine. Reducing the stigma of addiction. https://www.hopkinsmedicine.org/stigma-of-addiction/. Accessed March 1, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






