A review article in the Journal of Autoimmunity titled “Why Can't We Find a New Treatment for SLE?” describes the frustration and difficulties of diagnosing and treating patients with systemic lupus erythematosus (SLE).1 SLE is a complex, chronic autoimmune disease that mimics the symptoms of other diseases. SLE presents predominantly in women of child-bearing age. Its prevalence is approximately 1 in 2,000--similar to that of multiple sclerosis.2 There is higher incidence and prevalence, more severe course, and worse prognosis in persons of African American, Afro-Caribbean, and East Asian descent.3 In addition, older age at diagnosis, high disease activity at diagnosis, and greater overall disease activity are risk factors for a poor prognosis.4,5
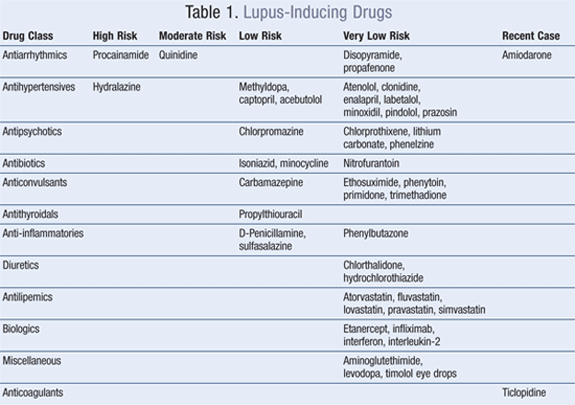
Disease manifestations range from skin involvement to multiple organ system damage. Half of patients with SLE experience organ damage within 5 years of diagnosis, generally involving the skin, kidneys, joints, nervous system, and hematopoietic organs. Early-stage SLE includes chronic, nonspecific symptoms such as fatigue, rash, joint pain, and fever. In a study of 150 patients suffering from SLE, arthralgias and cutaneous symptoms were present in nearly 75% of cases.5 These symptoms often present in a relapsing-remitting fashion and are exacerbated by sun exposure. Patients may experience sensitivity to light, headaches, depression, anxiety, heart complications, changes in weight, hair loss, swollen glands, cutaneous vasculitis, swelling of hands and feet, and anemia. Raynaud's disease, secondary to lupus, is characterized by an exaggerated vascular response to cold temperature or emotional stress and generally affects the fingers, toes, nose, and ears.6 Lupus may progress to include seizures, psychosis, renal failure, pulmonary hemorrhage, or sepsis.3,7 (TABLE 1).
The mortality rate with SLE is at least four times higher than that of the general population. Ten- and 20-year postdiagnosis survival rates are approximately 80% and 65%, respectively. Causes of mortality vary at different stages of the disease. Early mortality is often due to complications of active disease and infection, while later mortality is more often due to vascular events and organ failure.8
Pathogenesis
The exact etiology of SLE remains largely unknown.9 Genetic, hormonal, environmental, and immunoregulatory factors are all believed to contribute to the disease.3 Additionally, in relatively uncommon cases, lupus may be drug induced (TABLE 1).10 SLE nearly exclusively involves cumulative effects of several genes, predominately those involved in the immune response. New loci and genes that may lead to further discovery and development of disease-modifying therapies continue to be identified in the pathogenesis.11,12Along with gene discovery, multiple cellular abnormalities have been identified.3 Autoantibodies seen in SLE do not follow the expected patterns of biochemistry and can be detected in the serum of individuals years before the disease manifests clinically.11 In SLE, there is dysfunction among cellular components due to overproduction and underproduction of natural killer cells, B and T cells, antibodies, and proinflammatory cytokines.2
Research demonstrates that estrogen may be involved in the pathogenesis of SLE by affecting B-cell tolerance and contributing to autoimmune tendencies. Unraveling this myriad of molecular abnormalities is vital to the development of effective therapeutic agents. Despite these discoveries, few new therapies are proving effective.2,3,11
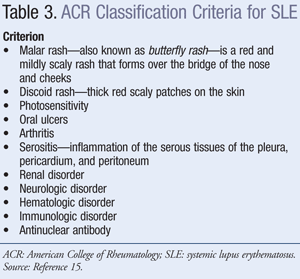
Diagnosis: A patient with two or more types of organ system involvement (TABLE 2) should be suspected of having SLE. However, several diseases are commonly confused with SLE, such as undifferentiated connective tissue disease, Sjögren's syndrome, antiphospholipid antibody syndrome, fibromyalgia with positive antinuclear antibody, idiopathic thrombocytopenia purpura, drug-induced lupus, early rheumatoid arthritis, and nonspecific vasculitis. The American College of Rheumatology criteria for classification of SLE were established for purposes of clinical studies; however, the diagnosis of SLE can be substantiated using these criteria. A patient is considered to have SLE when he or she presents with four out of 11 criteria presented in TABLE 3; however, a patient with fewer than four of these criteria could be diagnosed with SLE based on clinical judgment.13-15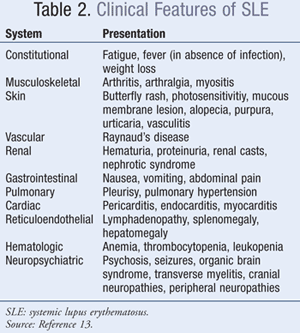
Treatment
The three drugs currently approved for treating SLE--aspirin, prednisone, and hydroxychloroquine--were approved during the Eisenhower administration, 1953-1961. Due to the potential for irreversible organ damage, treatments are generally managed by specialists (e.g., rheumatologists, immunologists), and routine, life-long monitoring of the disease and response to medications must occur. Depending on the drug regimen, monitoring may include albumin levels, complete blood counts with platelets, blood pressure, chest x-rays, funduscopic examinations, hepatitis B/C tests, liver function tests, serum creatinine levels, and urinalysis.16 Treatment guidelines are found in TABLE 4.

In treatment of severe SLE, B-cell-specific therapies are under investigation. Targets include B-cell tolerogens (abetimus), B-cell surface molecules (rituximab and epratuzumab), costimulatory molecules involved in B-cell-T-cell interaction (abatacept), and B-cell-activating factors and a proliferation-inducing ligand (belimumab, briobacept, atacicept).20
Regulatory authorities have not approved a new drug for lupus in more than 50 years. One such investigational drug, BenLysta (belimumab, formerly LymphoStat-B), is the first in a new class of BLyS antagonists to be marketed this year. Belimumab is a human monoclonal antibody against the human protein B-lymphocyte stimulator. In July 2009, Human Genome Sciences, a company that commercializes novel drugs for the treatment of hepatitis C, lupus, inhalation anthrax, and cancer, and GlaxoSmithKline PLC announced positive phase III study results in the primary end point of the 52-week, over-800-patient BLISS-52 study. Modest but statistically significant results demonstrated effectiveness in active disease. A reduction in the rates of flares, an increase in time to first flare, and a reduction in the use of prednisone were found in at least one of the treatment groups, belimumab 1 mg/kg or 10 mg/kg. Belimumab and placebo treatment groups had comparable rates of death, adverse events, infections, and laboratory abnormalities, yet the belimumab groups had modestly increased serious or severe infusion reactions.21,22
Educating Patients
Pharmacists play a vital role in educating and supporting patients with SLE. Patients newly diagnosed with SLE and their families often face anxiety over the complexity of the disease. Learning coping techniques and how to recognize flares and manifestations, as well as psychological support, is essential.13 Pharmacists can provide valuable disease state management services and referrals to patients with SLE in the areas of nutrition, osteoporosis, hyperlipidemia, cardiovascular disease, mental health, and renal impairment. In addition, patients should be thoroughly counseled on medication therapies. For example, patients on glucocorticoid treatment should be warned that irreversible damage can result from prolonged use of high-dose corticosteroids. Osteoporotic fractures, coronary artery disease, and cataracts are associated with cumulative prednisone doses. Avascular necrosis and stroke are associated with high prednisone doses, and cognitive dysfunction is associated with pulse methylprednisolone.4 For those with renal impairment, pharmacists should suggest that patients consult a nephrologist to discuss potential therapies, such as an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.13
Women of childbearing age should be educated on the teratogenic effects of several medications used to treat SLE and the potential need for contraception. For patients with SLE, routine health maintenance is especially important and includes gynecologic assessments, dental care, ophthalmologic examinations, and vaccinations. Each patient with SLE, particularly one receiving an immunosuppressive agent, should receive an inactivated influenza, pneumococcal, hepatitis B, and Haemophilus influenzae vaccination.13,15
In addition, pharmacists can assist patients in the prevention and treatment of Raynaud's disease secondary to lupus. These blood vessel spasms can be managed by lifestyle changes such as avoiding cold temperatures, caffeine, and nicotine; wearing warm clothing; as well as avoiding drugs that cause vasoconstriction and OTC medications such as decongestants, antimigraine medications containing ergotamine (e.g., Imitrex), herbal preparations containing ephedra, birth control pills, and the blood pressure medication clonidine (Catapres).23 When nonpharmacologic management is not sufficient, vasodilatory medications, such as calcium channel blockers, may be suggested. Blood pressure should be closely monitored when therapy is initiated.6
Patients may ask pharmacists about rumors claiming that artificial sweeteners such as aspartame (found in NutraSweet and Equal) can cause lupus. While patients have experienced idiosyncratic adverse effects such as headaches and insomnia, there does not appear to be a link between artificial sweeteners and lupus.24
Conclusion
Although long-term survival over the last several decades has improved, morbidity and complications from SLE remain significant.16 Additional research and new treatments remain critically needed. The complexity and unpredictability of the disease continue to present challenges for patients and health care providers, necessitating education and support for patients with this life-long, often progressive disease.References
1. Eisenberg R. Why can't we find a new treatment for SLE J Autoimmun. 2009;32:223-230.
2. Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303-306.
3. Tsokos GC, Kammer GM. Molecular aberrations in human systemic lupus erythematosus. Mol Med Today. 2000;6:418-424.
4. Zonana-Nacach A, Barr SG, Magder LS, Petri M. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum. 2000;43:1801-1808.
5. Heinlen LD, McClain MT, Merrill J, et al. Clinical criteria for systemic lupus erythematosus precede diagnosis and associated autoantibodies are present before clinical symptoms. Arthritis Rheum. 2007;56:2344-2351.
6. Drugs that aggravate or improve Raynaud's phenomenon. Pharmacist's Letter/Prescriber's Letter.
7. Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases [review]. Immunity. 2006;383-392.
8. Abu-Shakir M, Urowitz MB, Gladman DD, Gough J. Mortality studies in systemic lupus erythematosus: results from a single enter. J Rheumatol. 1995;22:1259-1264.
9. McClain MT, Arbuckle MR, Heinlen LD, et al. The prevalence, onset and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum. 2004;50:1226-1232.
10. Borchers AT, Keen CL, Gershwin ME. Drug-induced lupus. Ann NY Acad Sci. 2007;1108:166-183.
11. Crispín JC, Liossis SN, Kis-Toth K, et al. Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol Med. 2010;16:47-57.
12. Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526-1533.
13. American College of Rheumatology. Guidelines for referral and management of systemic lupus erythematosus in adults. Arthritis Rheum. 1999;42:1785-1796.
14. AHRQ. Systemic lupus erythematosus (SLE). Wiley Interscience. John Wiley & Sons; 2007. www.guidelines.gov. Accessed July 25, 2010.
15. Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271-1277.
16. Yazdany J, Panopalis P, Gillis JZ, et al. A quality indicator set for systemic lupus erythematosus. Arthritis Rheum. 2009;61:370-377.
17. Natural Comprehesive Medicines Database. www.naturaldatabase.com/(S(
18. Petri MA, Lahita RG, Vollenhoven RF, et al. Effects of prasterone on corticosteroid requirements of women with systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2002;46:1820-1829.
19. Clark WF, Parbtani A, Huff MW, et al. Flaxseed: a potential treatment for lupus nephritis. Kidney International. 1995;48:475-480.
20. Mo Yin M. The immunological basis of B-cell therapy in
systemic lupus erythematosus. Int J Rheum Dis [serial online]. March 2010;13:3-11. Academic Search Premier, Ipswich: MA. Accessed May 31, 2010.
21. Human Genome Sciences. Human Genome Sciences and GlaxoSmithKline announce positive phase 3 study results for Benlysta. www.hgsi.com/latest/human-
22. Navarra S, Guzman R, Gallacher A, et al. Belimumab, a BLyS-specific inhibitor, reduced disease activity, flares and prednisone use in patients with active SLE: efficacy and safety results from the phase 3 BLISS-52 study. Arthritis Rheum. 2009;60:3859-3861.
23. Raynaud's: the big chill for fingers and toes. Harvard's Heart Letter. 2010:3.
24. The safety of artificial sweeteners. Pharmacist Letter/Prescriber's Letter. 2006;22:220212.
25. Alarcon GS, McGwin G, Bertoli AM, et al. LUMINA Study Group. Effects of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis. 2007;66:1168-1172.
26. Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effects of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus. 2006;15:577-583.






