US Pharm. 2013;38(2):20-23.
ABSTRACT: Antiarrhythmic therapy for atrial fibrillation comprises a broad range of medications that are used to prevent conversion from normal sinus rhythm to atrial fibrillation, as well as to control symptoms. These medications, although effective, require extensive monitoring and patient education. The antiarrhythmics most commonly used to maintain normal sinus rhythm in atrial fibrillation patients are the class IC agents flecainide and propafenone and the class III agents amiodarone, dronedarone, sotalol, and dofetilide. Recommended monitoring parameters include renal and hepatic function, drug interactions, QT prolongation, and exacerbation of heart failure. Patient education should include drug interactions, adverse effects, and recommendations for laboratory monitoring. Community pharmacists are in a unique position to provide guidance on these medications to patients and providers.
Atrial fibrillation affects more than 2 million people in the United States, making it the most common atrial arrhythmia.1 It also accounts for one-third of hospitalizations for cardiac arrhythmias. This high prevalence is likely due to a combination of factors, including the increased incidence of chronic heart disease and the aging of the population. The median age of patients with atrial fibrillation is 75 years, with 70% of patients aged between 65 and 85 years.1 Common heart conditions that can lead to or exacerbate atrial fibrillation include heart failure (HF), coronary artery disease (CAD), cardiac surgery, and valvular disease.
Atrial fibrillation is caused by ectopic foci that arise in the atria, commonly after structural heart damage. These foci cause the atria to beat irregularly and out of coordination with the ventricles. Chronic atrial fibrillation can adversely affect a patient’s quality of life; most patients experience some degree of palpitation, dizziness, and dyspnea. More severe symptoms can occur when abnormal signals transmit to the ventricles, causing a rapid ventricular response that can result in hypotension, tachycardia, syncope, and chest pain. Another serious complication of atrial fibrillation is the higher risk of stroke or transient ischemic attack (TIA) due to a cardioembolic thrombus.
Treatment Goals and Strategies
The treatment goals for a patient with atrial fibrillation are to control the ventricular response and prevent thromboembolic complications.2 The need for long-term anticoagulation to reduce the risk of stroke and TIA is based on the patient’s risk level as measured by the CHADS2 (Cardiac failure, Hypertension, Age, Diabetes, Stroke Doubled) scoring system. Risk factors included in CHADS2 are HF, hypertension, age greater than 75 years, diabetes, and previous history of stroke or TIA. Patients with no CHADS2 risk factors are considered to be at low risk for thromboembolic events and should not receive anticoagulant therapy or aspirin monotherapy. Patients with a CHADS2 score of 1 or higher have a moderate-to-high risk of thromboembolism, and treatment with warfarin or one of the new anticoagulants, such as dabigatran, rivaroxaban, or apixaban, is recommended.2
Strategies to control the ventricular response include rhythm control and rate control. The rate-control method allows the atria to continue to fibrillate, and pharmacotherapy (commonly, beta-blockers, nondihydropyridine calcium channel blockers, and digoxin) is used to control the ventricular rate.1 The rhythm-control strategy utilizes a pharmacologic or nonpharmacologic modality to restore the atria to normal sinus rhythm, after which pharmacologic therapy is used to maintain normal sinus rhythm.
While rhythm control offers the advantage of increased cardiac output due to preservation of synchronized atrial contraction, no studies have demonstrated a benefit in clinical outcomes versus rate control. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) was a landmark randomized, multicenter trial of rate control versus rhythm control in 4,060 patients at high risk for stroke or death. AFFIRM found no significant difference between rhythm control and rate control in mortality incidence over 5 years (hazard ratio 1.15; 95% CI 0.99-1.34), but higher rates of hospitalization and adverse effects were seen in the rhythm-control group.3
Although rhythm control with antiarrhythmic therapy does not confer a mortality benefit over rate control, it is a mainstay of therapy for atrial fibrillation patients, especially those who remain symptomatic despite adequate rate control.4 Antiarrhythmic agents are categorized according to the Vaughan Williams classification: class I, sodium channel blockers; class II, beta-blockers; class III, potassium channel blockers; and class IV, calcium channel blockers.5 This review will focus on the use of class IC and III agents for maintenance of normal sinus rhythm in patients with atrial fibrillation.
Class IC Agents
The class I antiarrhythmic agents are sodium channel antagonists, with the class IC agents having a slow ion-channel dissociation and classes IA and IB having intermediate and fast dissociation kinetics, respectively.1 Class IC antiarrhythmic medications are proarrhythmic, and their use should be limited to patients without structural heart disease. The Cardiac Arrhythmia Suppression Trial (CAST) was designed to determine whether the class IC agents encainide, flecainide, and moracizine reduced the incidence of ventricular ectopy and sudden cardiac death after myocardial infarction (MI) in 1,498 patients.6 However, CAST was discontinued prematurely because of an increased incidence of mortality from arrhythmias, acute MI with shock, or congestive HF in the encainide and flecainide groups.6 Therefore, the use of class IC agents, including flecainide and propafenone (TABLE 1), should be limited to patients without left ventricular hypertrophy, CAD, and HF.1 These agents are generally less efficacious than other antiarrhythmics; approximately 23% of patients maintain normal sinus rhythm at 1 year.1
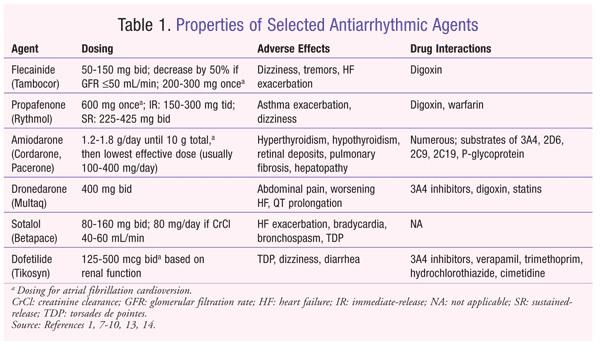
Flecainide (Tambocor): Flecainide is a potent sodium channel blocker that may be used in atrial fibrillation patients without structural heart disease.1,7 For atrial fibrillation maintenance, flecainide is generally dosed at 50 to 150 mg orally twice daily, with a dose decrease of 50% if the glomerular filtration rate is 50 mL/min or less; plasma levels are monitored closely. It also may be taken as a single oral loading dose of 200 to 300 mg for atrial fibrillation conversion. Plasma levels of flecainide may be monitored in the inpatient setting, with a goal trough level between 0.2 and 1.0 mcg/mL. Common adverse effects include dizziness, tremor, and HF exacerbation. If flecainide is used concomitantly with digoxin, the digoxin dose should be reduced by approximately 25%.
Propafenone (Rythmol): In addition to its sodium channel–blocking activity, propafenone has significant beta-blocking activity that may be beneficial in the management of atrial fibrillation.1,8 Typical dosing for the immediate-release formulation ranges from 150 to 300 mg orally two to three times daily. The extended-release formulation is dosed at 225 to 425 mg orally twice daily. The dose should be reduced in patients with hepatic or renal dysfunction, which will affect propafenone’s metabolism and excretion, respectively. Propafenone also may be taken as an oral loading dose of 600 mg for atrial fibrillation conversion. Adverse effects include unusual taste, asthma exacerbation, and dizziness. Digoxin and warfarin doses should be decreased with the use of propafenone.
Class III Agents
Class III agents are potassium channel blockers that exert their effects by prolonging the action potential. This leads to prolongation of the QT interval, which increases the risk of torsades de pointes (TDP), a potentially lethal ventricular arrhythmia. Common class III agents used for maintenance of normal sinus rhythm include amiodarone, dronedarone, sotalol, and dofetilide (TABLE 1).
Amiodarone (Cordarone, Pacerone): Amiodarone is a potent antiarrhythmic that has properties of all four Vaughan Williams classes (sodium channel blocker, potassium channel blocker, calcium channel blocker, beta-blocker).1,9 Amiodarone and dofetilide are the only two antiarrhythmic agents that do not increase mortality in HF patients. Amiodarone is safe for patients with structural heart damage and generally is considered more efficacious for maintaining normal sinus rhythm than other antiarrhythmics; rates of 62% to 69% at 1 year have been reported.1,3 The IV formulation has strong beta-blocking activity, whereas the oral formulation has more potassium channel–blocking activity. Because of its large volume of distribution (Vd) (approximately 60 L/kg), amiodarone requires a 10-g loading dose (oral or IV) before the dose is decreased to the lowest effective dose. Amiodarone inhibits numerous CYP enzymes, including 3A4, 2D6, 2C9, 2C19, and P-glycoprotein. Therefore, drug interactions and dose adjustments should be monitored closely when amiodarone is initiated or discontinued. Medications affected include digoxin, warfarin, and hydroxymethyl glutaryl coenzyme A reductase inhibitors (especially simvastatin).
Because of amiodarone’s iodine moiety and affinity for adipose tissue, use of the drug will lead to numerous organ-system toxicities, including hypothyroidism, hyperthyroidism, retinal deposits, inflammatory hepatopathy, and pulmonary fibrosis.1,9 Most of these adverse effects occur with doses exceeding 400 mg per day, which is the basis for the recommendation to reduce amiodarone to the lowest effective dose (commonly 200 mg daily for atrial fibrillation). Pulmonary-function tests, thyroid-function tests, liver-function tests, and chest radiographs are recommended at baseline, at 3, 6, and 12 months, and then annually thereafter to monitor for toxicity. An eye examination should be performed annually. Because of amiodarone’s large Vd and slow rate of elimination (mean half-life of 58 days; range 15-142 days), antiarrhythmic effects will persist for weeks or months after the drug is discontinued.
Dronedarone (Multaq): Dronedarone is a modified analogue of amiodarone that possesses similar properties of all four Vaughan Williams classes.1,10 Dronedarone is generally less efficacious than amiodarone, but has a lower incidence of organ toxicities.11 It is administered orally strictly at 400 mg twice daily. Contraindications to therapy include New York Heart Association functional class IV HF or recent HF exacerbation, severe hepatic impairment, second- or third-degree heart block, or heart rate under 50 beats per minute. Dronedarone also is not recommended for patients who are in permanent atrial fibrillation with no cardioversion planned.12 Dronedarone is not associated with the multitude of toxicities occurring with amiodarone, but adverse effects include abdominal pain, nausea, diarrhea, QT prolongation, and HF exacerbation. There have been recent reports of hepatic toxicity, including severe liver damage. Because dronedarone is a CYP3A4 substrate, it should be avoided with concurrent CYP3A4 inhibitors, such as ketoconazole, voriconazole, cyclosporine, and clarithromycin. Dronedarone will increase serum concentrations of digoxin and statins.
Sotalol (Betapace): Although sotalol has effects on the potassium channel, its beta-blocking activity is more potent.1,13 It is ineffective for conversion of atrial fibrillation and should be used only to maintain normal sinus rhythm. Sotalol is a modestly effective antiarrhythmic; approximately 37% of patients maintain normal sinus rhythm at 1 year, similar to the efficacy of class IC agents.1 Sotalol is dosed at 80 to 160 mg orally twice daily, and it is recommended to reduce the frequency to once daily if creatinine clearance (CrCl) is between 40 and 60 mL/min. Sotalol is contraindicated for atrial fibrillation when CrCl is less than 40 mL/min. Many of sotalol’s adverse effects are due to the drug’s beta-blocking activity, including HF exacerbation, bradycardia, atrioventricular block, and bronchospasm. Sotalol should be initiated in the hospital setting in order to monitor for QT-interval prolongation, because there is a risk of TDP within the first few days of initiation.
Dofetilide (Tikosyn): Dofetilide, a pure class III potassium channel blocker, is effective for both atrial fibrillation cardioversion and maintenance of normal sinus rhythm.1,14 It is the only other medication, besides amiodarone, that does not increase mortality in patients with HF. The risks of QT prolongation and TDP upon initiation are high (4%), however, so dofetilide must be initiated in the hospital setting. It is generally efficacious, with approximately 58% of patients maintaining normal sinus rhythm at 1 year.1 The dose of dofetilide ranges from 125 to 500 mcg orally twice daily based on CrCl. Use is contraindicated if CrCl is less than 20 mL/min. The first five doses must be given in the hospital, with ECGs performed 2 to 3 hours after each dose to monitor for QT prolongation. Subsequent dose adjustments are based on these QT measurements. In order to prescribe and dispense dofetilide, the physician, hospital, and pharmacy must be registered with the manufacturer. Drug interactions that affect dofetilide concentrations include CYP3A4 inhibitors and medications that inhibit active tubular secretion in the kidneys.
The Pharmacist’s Role
Pharmacists in the community setting are ideally situated to participate in the management of antiarrhythmic therapy in patients with atrial fibrillation. As evidenced by the multitude of interactions, adverse effects, and monitoring parameters listed above, there are many opportunities for pharmacists to provide patient education and to advise prescribers. The initiation or discontinuation of medications that affect concentrations of antiarrhythmic therapy is common, and potentially serious effects could ensue without involvement by the pharmacist. In addition to medication education, the pharmacist also can discuss with patients the indication and purpose of antiarrhythmic medications in the prevention of atrial fibrillation events. In this manner, pharmacists can be involved in the overall management of patients with atrial fibrillation.
REFERENCES
1. Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused
updates incorporated into the ACC/AHA/ESC 2006 guidelines for the
management of patients with atrial fibrillation: a report of the
American College of Cardiology Foundation/American Heart Association
Task Force on Practice Guidelines developed in partnership with the
European Society of Cardiology and in collaboration with the European
Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101-e198.
2. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for
atrial fibrillation: antithrombotic therapy and prevention of
thrombosis, 9th ed: American College of Chest Physicians Evidence-Based
Clinical Practice Guidelines. Chest. 2012;141(suppl 2):531S-575S.
3. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
4. Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 2012;125:381-389.
5. Vaughan Williams EM. Classification of antidysrhythmic drugs. Pharmacol Ther B. 1975;1:115-138.
6. Preliminary report: effect of encainide and flecainide on
mortality in a randomized trial of arrhythmia suppression after
myocardial infarction. N Engl J Med. 1989;321:406-412.
7. Tambocor (flecainide) product information. Northridge, CA: 3M Pharmaceuticals; June 1998.
8. Rythmol (propafenone) product information. Research Triangle Park, NC: GlaxoSmithKline; June 2011.
9. Cordarone (amiodarone) product information. Philadelphia, PA: Wyeth Pharmaceuticals Inc; October 2011.
10. Multaq (dronedarone) product information. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; September 2012.
11. Le Heuzey JY, De Ferrari GM, Radzik D, et al. A short-term,
randomized, double-blind, parallel-group study to evaluate the efficacy
and safety of dronedarone versus amiodarone in patients with persistent
atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol. 2010;21:597-605.
12. Connolly SJ, Camm AJ, Halperin JL, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268-2276.
13. Betapace (sotalol) product information. Wayne, NJ: Bayer Healthcare Pharmaceuticals; October 2010.
14. Tikosyn (dofetilide) product information. New York, NY: Pfizer Inc; February 2011.
To comment on this article, contact rdavidson@uspharmacist.com.





