US Pharm. 2022;47(12):37-42.
ABSTRACT: The incidence and severity of influenza infections vary from year to year, and obtaining the annual influenza vaccine remains the best approach to reducing the incidence of severe infections, hospitalization, influenza-related complications, and death. Recently, the CDC issued clinical practice recommendations for the upcoming 2022–2023 influenza season, including the use of four FDA-approved antiviral drugs for the treatment and prevention of influenza. Pharmacists are well poised to educate patients about influenza, including modes of transmission, clinical presentation, and available treatments; they can also give patients pertinent information regarding factors that confer a greater risk of influenza-related complications and suggest measures to prevent or lessen the severity of these infections.
Influenza is a highly contagious acute respiratory infection caused by influenza virus A, B, or C, with type C generally causing mild illness. Outbreaks and epidemics occur globally and usually align with the winter season (December to March, in the United States). The CDC states that the severity of the influenza virus in the U.S. can fluctuate extensively from year to year and is contingent upon several factors, including the characteristics of circulating viruses, the timing of the season, the efficacy of the vaccine coverage, and the number of people vaccinated during that particular season.1 According to the CDC, influenza activity during the 2021–2022 U.S. influenza season was lower than prepandemic activity despite increased reporting and testing—possibly because of COVID-19 precautions—and the 2021–2022 season was considered mild compared with other influenza seasons.2 Moreover, the CDC estimated that from early October 2021 through mid-June 2022, influenza infections contributed to an estimated 8 million to 13 million symptomatic illnesses, 3.7 million to 6.1 million medical visits, 82,000 to 170,000 hospitalizations, and 5,000 to 14,000 deaths.2
Annual influenza vaccination is the best way to lessen the number of severe influenza infections and related health complications. For the 2022–2023 influenza season, the CDC recommends the use of four FDA-approved antiviral drugs to treat influenza: oseltamivir, zanamivir, peramivir, and baloxavir marboxil.2 A number of clinical trials show that these antivirals, when initiated promptly after diagnosis, have the potential to lessen influenza symptoms, shorten the duration of illness, and prevent serious influenza-related complications.2
Signs and Symptoms of Influenza
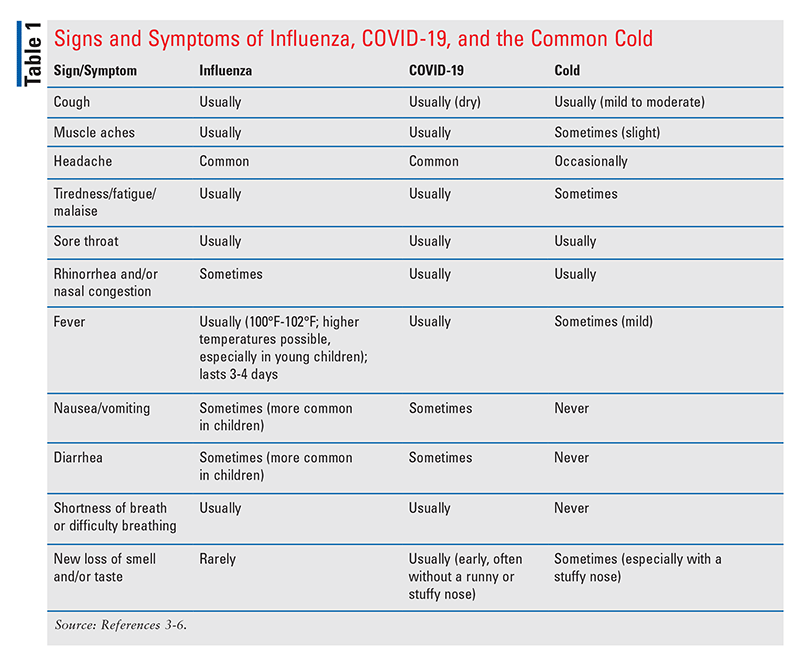
Pharmacists can perform a valuable service by educating patients about modes of transmission, signs and symptoms of influenza, patient populations at greater risk for influenza-related complications, best means of prevention, possible treatments, and when to seek medical attention. Pharmacists can also be instrumental in guiding patients in the selection and appropriate use of nonprescription medications available for the management of mild-to-moderate influenza symptoms. These medications, including decongestants, antihistamines, expectorants, cough suppressants, and antipyretics/analgesics, are available as single-entity or multi-ingredient products. In order to make accurate clinical assessments tailored to patient need, pharmacists must be able to differentiate between the typical symptoms and signs of influenza, COVID-19 infection, and the common cold (TABLE 1).3-6
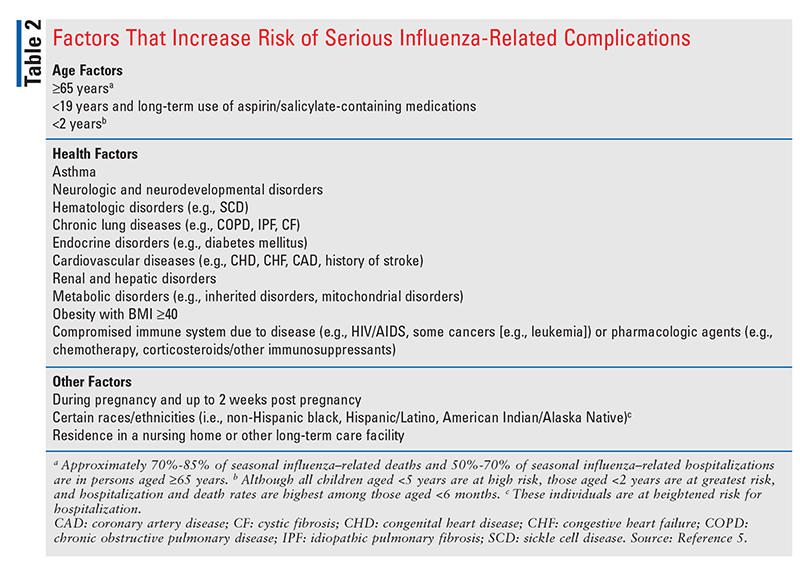
Factors That Increase Risk of Influenza-Related Complications
Although anyone can develop complications from the influenza virus, the CDC advises that certain patient populations are more vulnerable to developing influenza-related complications, are at greater risk for hospitalization, and have higher mortality rates. According to the CDC, infants and elderly patients have the highest mortality rates.5 See TABLE 2 for a list of factors that increase the risk of serious complications.5
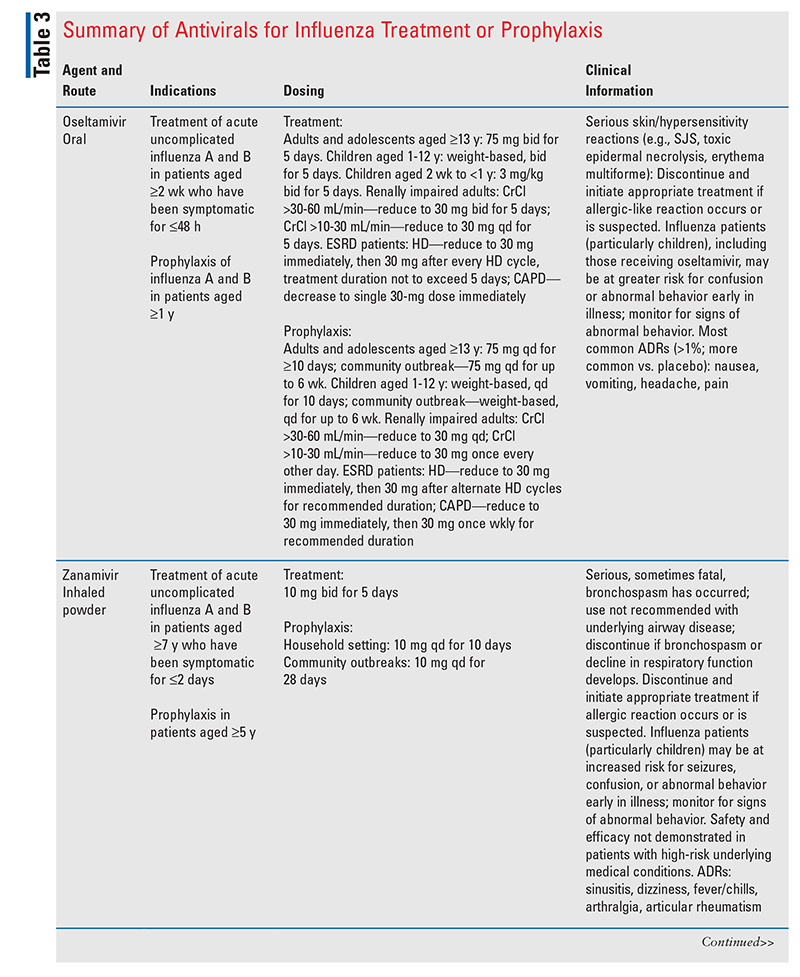
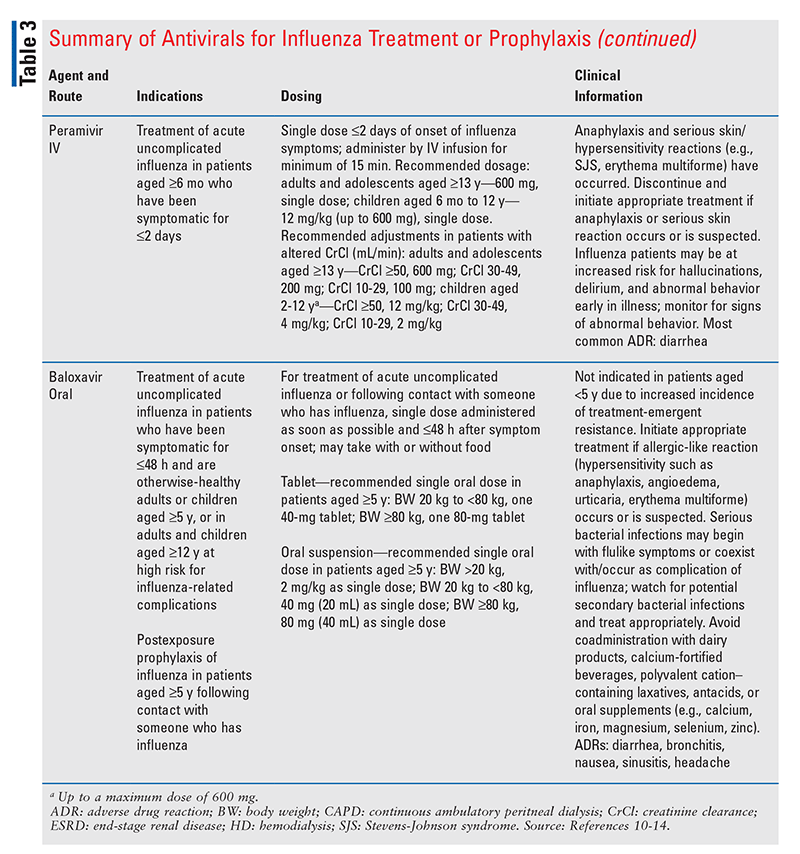
Influenza Management and Prevention
Clinical data from randomized, controlled trials, meta-analyses of randomized, controlled trials, and observational studies conducted during past influenza seasons and the 2009 H1N1 pandemic demonstrate that antiviral therapy has both clinical and public-health benefits in reducing the burden of illness and severe outcomes of influenza.7
Classes of antivirals available in the U.S. for influenza treatment and chemoprophylaxis include the neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) and an endonuclease inhibitor (baloxavir marboxil).2 In a CDC report summarizing U.S. influenza activity between October 3, 2021, and June 11, 2022, all of the influenza viruses collected and tested by the CDC for antiviral resistance were found to be susceptible to zanamivir, and the majority (>99%) were susceptible to oseltamivir, peramivir, and baloxavir marboxil.2
The neuraminidase inhibitors selectively block an enzyme conserved within all influenza viruses to prevent virus particles from exiting host cells, consequently inhibiting person-to-person viral transmission.8 Neuraminidase inhibitors also curtail symptom duration, possibly by decreasing the viral load, preventing the spread and release of cytokines, and reducing the risk of complications.8 The novel one-dose oral antiviral drug baloxavir marboxil, which is a selective inhibitor of influenza cap-dependent endonuclease, blocks proliferation of the influenza virus by impeding the initiation of messenger RNA synthesis.9 Key information about these antivirals is given in TABLE 3.10-14
The CDC notes that complications of influenza include bacterial infections such as pneumonia, otitis media, sinus infections, and dehydration, and that influenza may also exacerbate certain medical conditions (e.g., congestive heart failure, asthma, diabetes mellitus).15 Bacterial coinfection or development of complications may necessitate the use of antibiotics in patients with influenza, when warranted.15
Recommendations for Antiviral Use in the 2022–2023 Season
According to the CDC, early diagnosis of influenza can decrease the inappropriate use of antibiotics and enable the prompt administration of an appropriate antiviral tailored to patient need.14 The CDC makes the following antiviral recommendations for treatment and chemoprophylaxis of influenza: 1) For hospitalized patients with suspected or confirmed influenza, initiation of antiviral treatment with oral or enterically administered oseltamivir is advised as soon as possible; 2) for outpatients with complications or progressive disease and suspected or confirmed influenza (e.g., pneumonia, exacerbation of underlying chronic medical conditions), initiation of antiviral treatment with oral oseltamivir is advised as soon as possible; and 3) for outpatients with suspected or confirmed uncomplicated influenza, the agents oral oseltamivir, inhaled zanamivir, IV peramivir, or oral baloxavir may be used for treatment, within the parameters of contraindications and approved age groups. In one randomized, controlled trial, baloxavir had superior efficacy over oseltamivir in adults and adolescents with influenza B infection.14,16
The CDC states that considerations regarding initiation of antiviral treatment for patients with suspected influenza should not wait for laboratory confirmation of infection.14 The use of early empirical antiviral treatment should be considered in non–high-risk outpatients with suspected influenza (e.g., fever with cough or sore throat), centered on clinical judgment, if treatment can be initiated within 48 hours of onset.14 The possibility of COVID-19 and other flulike illnesses should also be considered.14
The CDC provides guidance about the cocirculation of influenza viruses and COVID-19. Empirical antiviral treatment of influenza is recommended as soon as possible for the following priority groups: 1) outpatients with severe, complicated, or progressive respiratory illness and 2) outpatients at greater risk for influenza complications who present with any acute symptoms of respiratory illness (with or without fever).14
More in-depth information about the CDC recommendations on the use of antivirals for influenza treatment is available online in the CDC’s summary for clinicians.14
Clinical Data on Antiviral Agents
In a recent review and meta-analysis, researchers compared the efficacy and safety of neuraminidase inhibitors and the endonuclease inhibitor zanavir for treatment of seasonal influenza in healthy adults and pediatric patients.8 The network meta-analysis of 26 randomized clinical trials involving 11,897 patients found that antiviral agents had significantly greater efficacy versus placebo in lessening the duration of influenza symptoms. The use of zanamivir was linked to the shortest time to alleviation of influenza symptoms, and baloxavir was associated with a lower incidence of influenza-related complications compared with other treatments.8
In August 2022, the FDA expanded the indications for baloxavir marboxil and approved a supplemental New Drug Application for treatment of acute uncomplicated influenza in otherwise-healthy children aged 5 years to <12 years who have been symptomatic for no more than 48 hours.17 This approval marks the first single-dose oral influenza medication for children in this age group. The FDA also approved this agent for prevention (postexposure prophylaxis) of influenza in patients aged 5 years to <12 years following contact with someone with influenza. Approval was based on results from two phase III studies: miniSTONE-2 and BLOCKSTONE. The miniSTONE-2 trial evaluated baloxavir versus oseltamivir in otherwise-healthy children aged 5 years to <12 years with influenza infection and displaying influenza symptoms for no more than 48 hours. The average time to relief of signs and symptoms in influenza-infected participants was 138 hours (95% CI, 117; 163) and 150 hours (95% CI, 115; 166) for those who received baloxavir or oseltamivir, respectively.18
BLOCKSTONE compared baloxavir with placebo as preventive treatment for adults and children living in the same household as an individual with influenza.19 Baloxavir was found to have a statistically significant prophylactic effect on influenza after a single oral dose: The risk of an individual aged 12 years or older developing influenza after exposure to an infected household member was reduced by 90% versus placebo, and the proportion of household members aged 12 years or older who developed laboratory-confirmed clinical influenza was 1.3% in the baloxavir versus 13.2% in the placebo group.19 Adverse events reported in at least 5% of baloxavir-treated patients aged 5 to 11 years included vomiting and diarrhea (each, 5%).17
A cross-sectional study was conducted in pediatric (ages 1-18 years) outpatients at high risk for influenza complications who were diagnosed with influenza during the 2016–2019 influenza seasons. The primary outcome was the dispensing of an influenza antiviral within 2 days of influenza diagnosis. It was determined that only 58% of these 274,213 patients received antiviral treatment.20 The investigators concluded that more research is needed to identify and address barriers to the use of antivirals in this high-risk patient population.20
A large study of rheumatoid arthritis (RA) patients with influenza infection diagnosed in an outpatient setting examined whether healthcare-resource use and costs differed between patients with RA and influenza who received antiviral therapy and matched patients with RA and influenza who did not.21 In this real-world sample, antivirals reduced the healthcare burden associated with outpatient and emergency-department visits over 28 days of follow-up. The researchers concluded that timely use of antiviral treatment after influenza diagnosis has the potential to reduce healthcare-resource use and lessen the economic burden in this patient population.21
Conclusion
As front-line healthcare providers, pharmacists can play a critical role in expanding patients’ awareness about the significance of diagnosing and treating influenza promptly per current CDC recommendations, ensuring the proper use of antivirals to reduce the duration and severity of influenza, and preventing or minimizing influenza-related complications. Through pharmacist intervention and patient education, patients will be equipped with sufficient knowledge to make informed decisions about their health and take measures to reduce the transmission of influenza. Valuable patient-education resources from the National Foundation for Infectious Diseases and the CDC are available online.22,23
Pharmacists can screen for potential drug-drug interactions and contraindications as well as counsel patients on the proper use of prescribed antivirals, including dosage, administration, duration of use, and potential adverse effects. They can also make recommendations about nonpharmacologic measures to alleviate influenza symptoms, such as staying home and resting; maintaining adequate hydration; and using a vaporizer or humidifier, saline nasal sprays, and nonmedicated nasal strips to relieve nasal congestion. During counseling, pharmacists should remind patients about the safety and efficacy of influenza vaccines and their value in preventing serious infections and reducing rates of hospitalization and/or mortality, as well as the importance of generally keeping up-to-date with recommended vaccinations for their age group.
REFERENCES
1. CDC. Disease burden of flu. www.cdc.gov/flu/about/burden/index.html. Accessed September 29, 2022.
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71(29):913-919.
3. WebMD. Flu or cold symptoms? www.webmd.com/cold-and-flu/flu-cold-symptoms. Accessed September 29, 2022.
4. CDC. Cold versus flu. www.cdc.gov/flu/symptoms/coldflu.htm. Accessed September 29, 2022.
5. CDC. About flu. www.cdc.gov/flu/about/index.html. Accessed September 29, 2022.
6. World Health Organization. Coronavirus disease (COVID-19): similarities and differences between COVID-19 and influenza. www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-similarities-and-differences-with-influenza. Accessed September 29, 2022.
7. Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011-2016. Clin Infect Dis. 2018;66(7):1035-1041.
8. Liu JW, Lin SH, Wang LC, et al. Comparison of antiviral agents for seasonal influenza outcomes in healthy adults and children: a systematic review and network meta-analysis. JAMA Netw Open. 2021;4(8):e2119151.
9. Heo YA. Baloxavir: first global approval. Drugs. 2018;78(6):693-697.
10. Tamiflu (oseltamivir) product information. South San Francisco, CA: Genentech, Inc; August 2019.
11. Xofluza (baloxavir marboxil) product information. South San Francisco, CA: Genentech, USA, Inc; August 2022.
12. Rapivab (peramivir) product information. Durham, NC: BioCryst Pharmaceuticals, Inc; January 2021.
13. Relenza (zanamivir) product information. Research Triangle Park, NC: GlaxoSmithKline; October 2021.
14. CDC. Influenza antiviral medications: summary for clinicians. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed September 29, 2022.
15. CDC. Key facts about influenza (flu). www.cdc.gov/flu/about/keyfacts.htm. Accessed September 29, 2022.
16. Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204-1214.
17. Businesswire. Genentech announces FDA approval of Xofluza to treat influenza in children aged five and older. www.businesswire.com/news/home/20220815005009/en. Accessed September 29, 2022.
18. Baker J, Block SL, Matharu B, et al. Baloxavir marboxil single-dose treatment in influenza-infected children: a randomized, double-blind, active controlled phase 3 safety and efficacy trial (miniSTONE-2). Pediatr Infect Dis J. 2020;39(8):700-705.
19. Ikematsu H, Hayden FG, Kawaguchi K, et al. Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med. 2020;383(4):309-320.
20. Antoon JW, Hall M, Feinstein JA, et al. Guideline concordant antiviral treatment in children at high-risk for influenza complications. Clin Infect Dis. 2022;ciac606.
21. Neuberger EE, To TM, Seetasith A, et al. Antiviral use and health care use among US patients with rheumatoid arthritis and influenza in three influenza seasons, 2016-2019. ACR Open Rheumatol. 2022;4(7):631-639.
22. National Foundation for Infectious Diseases. Are you at risk for serious flu-related complications? www.nfid.org/infectious-diseases/are-you-at-risk-for-flu-and-related-complications. Accessed September 29, 2022.
23. CDC. Flu: what to do if you get sick. www.cdc.gov/flu/treatment/takingcare.htm. Accessed September 29, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






