ABSTRACT: Since 2010, A1C, the glycosylated form of hemoglobin, has been recognized by diabetes mellitus (DM) clinical guidelines as a diagnostic tool. Three of the latest clinical guidelines on DM address diagnosis and management. One of the guidelines, the American College of Physicians’ guidance statement, has generated controversy because it has recommended higher target glycemic goals than the other recommendations. Limitations have been identified surrounding the use of A1C as a diagnostic aid in certain patient populations. Patient-specific and assay-specific interferences that exist can potentially result in inaccuracies in A1C readings.
A1C results from the nonenzymatic glycation of the amino (N)-terminal valine residue of hemoglobin A. This process is dependent on ambient glucose concentrations and occurs throughout the 120-day lifespan of the red blood cell (RBC). Therefore, A1C is used to measure glycemic control over the previous 3 months.1-3 A1C represents a weighted average, with approximately 50% of the value due to the mean blood glucose (BG) concentrations in the 30 days prior to sampling; BG concentrations from the previous 90 to 120 days make up about 10% of the final total A1C value.2
A1C testing gained acceptance as a diagnostic tool for diabetes mellitus (DM) in 2009, when it was formally endorsed by an International Expert Committee that was examining its role.4 It was later incorporated into American Diabetes Association (ADA) guidelines and was subsequently adopted by the World Health Organization.5,6
This shift in policy happened following the publication of the results of the Diabetes Control and Complications Trial (DCCT) and The United Kingdom Prospective Diabetes Study (UKPDS), which found that reductions in A1C resulted in decreased risk of microvascular complications in type 1 DM (T1DM) and type 2 DM (T2DM) patients, respectively.7,8 In the 1990s, the National Glycohemoglobin Standardization Program (NGSP) was developed to formalize A1C testing. The NGSP’s goal is to standardize A1C results to those of the DCCT trial, a process termed traceability.9 The International Federation of Clinical Chemistry (IFCC) Working Group on Hemoglobin A1C Standardization has developed reference methods to assure the traceability and standardization of values assigned to A1C test calibrators and/or control materials against those used in DCCT.10 The IFCC has also called for the reporting of A1C values as mmoL/moL rather than as a percentage, which is the reporting method used by the NGSP network and the United States. The following formula has been developed for converting between the NGSP and IFCC values10: NGSP = [0.09148*IFCC] + 2.152.
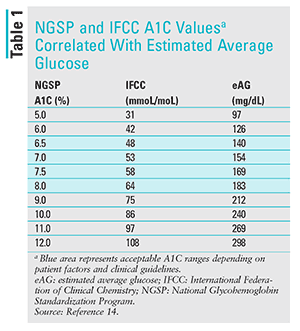
The ADAG (A1C-Derived Average Glucose) study found a strong correlation between the A1C and estimated average glucose concentrations.11,12 A change (either positive or negative) in A1C percentage of 0.5% is considered clinically significant.13 See TABLE 1.14
Three clinical guidelines published in 2018 that have addressed the utilization of A1C in the diagnosis and management of DM include the ADA Standards of Medical Care in Diabetes 2018, the Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) on the Comprehensive T2DM Management Algorithm-—2018 Executive Summary, and the guidance statement update from the American College of Physicians (ACP) on A1C targets for glycemic control with pharmacologic therapy for nonpregnant adults with T2DM.15-17 This article will review these recommendations, as well as limitations and interferences with A1C testing.
Recent A1C Guidelines
American Diabetes Association’s Summary of Revisions: Standards of Medical Care in Diabetes-—2018: In 2018, the ADA guidelines were updated to reflect potential limitations in A1C measurements due to hemoglobin variants, assay interferences, ethnicity, age, and conditions associated with altered RBC turnover, all of which may necessitate the use of an alternative form of diagnostic glucose testing. In the case of assay interference, marked disconcordance between measured A1C and observed plasma glucose concentrations should prompt an investigation into the presence of hemoglobin variants that may interfere with test results.5
The diagnosis of DM is made when the A1C values are >6.5% (48 mmoL/moL) based on an NGSP-certified test. Prediabetes is defined by an A1C of 5.7% to 6.4% (39-47 mmoL/moL). Patients with prediabetes should be tested yearly in order to determine whether they have converted to diabetic status. Plasma glucose concentrations are recommended over A1C testing for diagnosing T1DM patients who have overt symptoms of hyperglycemia, most of whom are pediatric patients. On the other hand, A1C, fasting plasma glucose, and 2-hour plasma glucose values obtained during oral glucose tolerance testing are equally beneficial in diagnosing T2DM in both younger and older patients.18
TABLE 2 identifies patient-specific factors that interfere with A1C testing.2,5,19-38 Plasma glucose concentrations (or other alternative glycemic assessments, such as fructosamine and glycated albumin) may be recommended over A1C testing in these patient populations.18
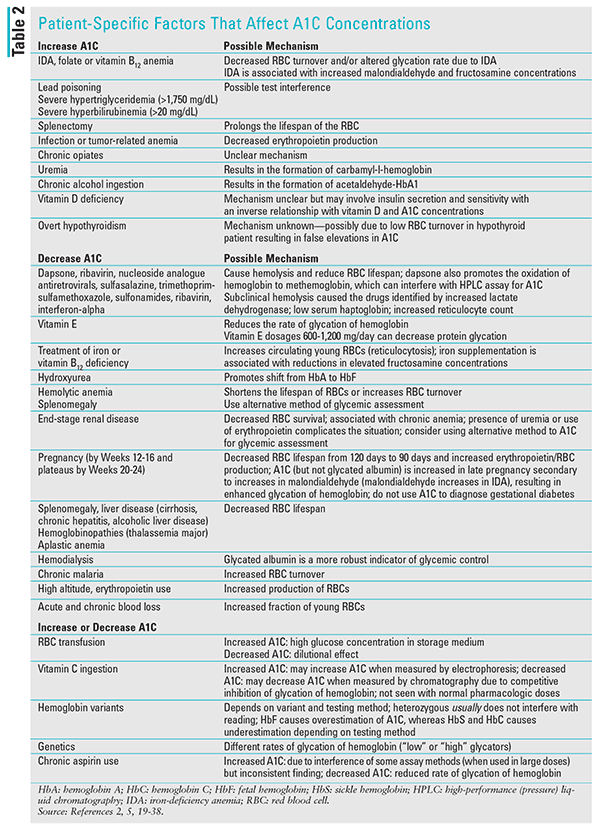
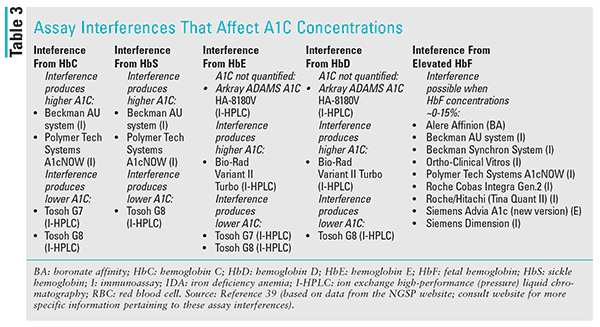
TABLE 3 lists a few common hemoglobin variants and hemoglobinopathies that interfere with A1C assays.40,41 A genome-wide association study involving approximately 160,000 people of European, African, East Asian, and South Asian ancestry recently found that there are 60 genetic variants that can influence A1C.42
In general, there are five different types of assay methods for measuring A1C: enzymatic, immunoassay, boronate affinity, ion-exchange high-performance liquid chromatography, and capillary electrophoresis.3,43 A current list of FDA-approved immunoassays is available.44 A review of variant hemoglobins interfering with A1C measurement has been published.45
Patient-specific factors that affect A1C concentrations are race and age. African Americans have higher A1C concentrations for any mean glucose concentration compared with non-Hispanic whites.18,46 These higher A1C concentrations may occur in the presence of similar fasting and postglucose load glucose concentrations.18 Glucose concentrations increase as glucose intolerance worsens.33 Similar findings have been observed when fructosamine and glycated albumin, which are alternative methods to assess glycemic status, are employed.18 Elevated A1C concentrations have also been found in other racial groups.47 African Americans’, Hispanics’, and Asians’ A1C concentrations are 0.37%, 0.27%, and 0.33%, respectively—higher compared with those of whites.25
With regard to age, A1C testing is not recommended to diagnose T1DM in pediatric patients.18
The ADA guidelines recommend that A1C testing be performed at least twice yearly in patients who have achieved stable glycemic control. For those patients who are not at goal or for whom therapy recently changed, quarterly A1C testing is recommended. The guidelines also caution that A1C does not measure glycemic variability or hypoglycemic risk, although hypoglycemia is less common among patients with A1C values of <7.0% to 7.5% (53-58 mmoL/moL).12 The ADA offers guidelines for initiating and escalating therapy based on A1C concentrations.48
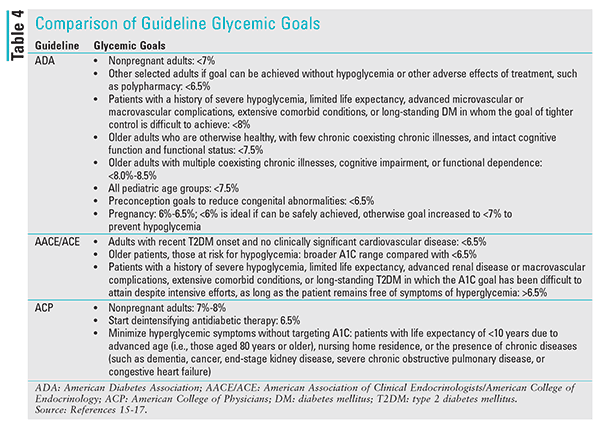
American Association of Clinical Endocrinologists and American College of Endocrinology Consensus Statement on the Comprehensive Type 2 Diabetes Management Algorithm (2018 Executive Summary): At the same time that the ADA released its guidelines on the management of DM, the AACE/ACE disseminated its recommendations.16 Similar to the ADA, AACE/ACE advises that A1C targets be individualized based on age, life expectancy, comorbid conditions, duration of DM, risk of hypoglycemia, or adverse consequences from hypoglycemia. However, if individualized A1C targets can be achieved safely, AACE/ACE identifies an A1C concentration of <6.5% as the optimal glycemic goal for patients with recent DM onset and no clinically significant cardiovascular disease. It also states that higher glycemic goals (>6.5%) may be appropriate for some patients (TABLE 4). These goals may change for the individual patient over time. Algorithm-based recommendations are provided for treatment options based on the initial A1C value.
American College of Physicians’ Guidance Statement on A1C Targets for Glycemic Control With Pharmacologic Therapy for Nonpregnant Adults With Type 2 Diabetes Mellitus: The most contentious of these diabetic guidelines are those issued by the ACP.17 The ACP reviewed six clinical practice guidelines from other organizations in which A1C goals were used to manage antidiabetic therapy.49-54 The group then released four guidance statements that recommended deintensification of hypoglycemic therapy for nonpregnant adults with T2DM. The ACP advocates that clinicians personalize goals for therapy based on the risk versus benefit of pharmacotherapy, treatment burden, and cost. It also recommends glycemic goals of 7% to 8% in most patients with T2DM. For patients who have achieved A1C concentrations of <6.5%, the guidance advises deintensification of drug therapy. This liberalization of glycemic control is advocated because, according to ACP, such low concentrations have not been associated with improved clinical outcomes but are related to increased cost, patient burden, and adverse events. Lastly, the ACP encourages clinicians to minimize hyperglycemic symptoms rather than target A1C concentrations in patients with a life expectancy of <10 years due to advanced age, nursing home residence, or the presence of chronic diseases (TABLE 4) because harm may outweigh benefit in these patients.
The authors of the guidelines argue that intensive glycemic control is associated with small absolute reductions in the risk of microvascular surrogate events (e.g., retinopathy on ophthalmologic examination or nephropathy defined by the presence of albuminuria) and that A1C concentrations of <7% have not consistently shown reductions in clinical microvascular events, such as loss or impairment of vision, end-stage renal disease, or painful neuropathy.17
Additionally, A1C targets of <7% (compared with 8%) were not associated with decreased deaths (either all-cause or cardiovascular-related deaths) or reductions in macrovascular events over a 5- to 10-year treatment period. Further, the authors point out that in all studies that randomized patients to more intensive therapy to lower the A1C, there were higher rates of adverse events, including death, compared with more liberal treatment goals. They caution that it takes a long time to achieve the benefits associated with more intensive glycemic control, and such restrictive regimens may be best for patients with life expectancy in excess of 15 years. Finally, the authors believe that there was insufficient evidence to evaluate clinical outcomes for A1C concentrations between 6.5% and 7.0%.
The Endocrine Society, ADA, AACE, the American Association of Diabetes Educators, and the Joslin Diabetes Center have openly criticized the loosening of glycemic goals.55-57 They take ACP to task because their recommendations contradict the findings of DCCT, UKPDS, ACCORD (Action to Control Cardiovascular Risk in Diabetes), ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation), and VADT (Veterans Affairs Diabetes Trial), all of which associated lower A1C concentrations with reduced microvascular complications.7,8,58-60 Further, ACP’s recommendations do not take into account the younger T2DM patient, who may be at greater risk for long-term complications, such as cardiovascular disease, retinopathy, amputations, and kidney disease, if the higher A1C targets are implemented. The ACP has also been faulted for failure to recognize the positive impact that newer classes of hypoglycemics, such as the sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists, have had on both improved cardiovascular outcomes and glycemic control while having a low risk of hypoglycemia. There is also concern that these more relaxed glycemic targets may become performance measures for health plans.55-57,61,62
Alternative Glycemic-Control Measures
In situations where measuring A1C may produce inaccurate results, such as in people of African, Mediterranean, or Southeast Asian descent or in any condition that shortens RBC survival or decreases mean RBC age, alternative approaches to assessing long-term glycemia may be preferred.22,63 Clinicians should consider the possibility of a test interference when the A1C is >15% or is at odds with other BG results.64 However, these alternative methods have not been standardized or correlated with long-term complications in DM, as has the A1C.3,65
Fructosamines are nonenzymatically glycated proteins. They represent all of the serum-stable proteins and reflect glycemic control over the previous 2 to 3 weeks.20,25 The fructosamine test is useful in patients with renal disease, but not in those with hypoproteinemic states, such as liver disease, nephrotic syndrome, or nephrosis, or in uremia and lipidemia. Fructosamine results are affected by concentrations of serum proteins and the presence of low-molecular-weight substances in the plasma.20,25
Glycated albumin also represents BG control over the previous 2 to 3 weeks. It may also be useful in assessing glycemic status during pregnancy, for patients on hemodialysis or receiving erythropoietin therapy, those with chronic kidney disease, those with severe hemoglobinopathies, and in patients with altered RBC lifespan or degrees of glycation.1,2,20,66 Similar to fructosamine, glycated albumin is unreliable in hypoproteinemic states, uremia, and lipidemia.20 There may be racial differences in measurements of glycated albumin, with blacks having increased concentrations compared with whites.65 It may be more useful than A1C in assessing postprandial hypoglycemia.25
Another substance, 1,5-anhydroglucitol, is also used to assess glycemic control because higher glucose concentrations competitively inhibit its renal absorption, resulting in an inverse relationship with BG concentrations.1 However, it is not useful in patients with renal failure or chronic liver disease and during pregnancy.20,25 It appears most useful in monitoring postprandial glycemic control.20
Continuous glucose monitoring is superior to A1C testing in assessing the degree of glycemic variability over time, as well as in evaluating episodes of hypoglycemia. It can be used to confirm the validity of A1C testing.25
Conclusion
The pharmacist’s role in improving therapeutic outcomes for diabetic patients is well documented.67-70 Despite the current controversy surrounding the clinical guidelines, the pharmacist can play a major role by individualizing therapy. As drug experts, pharmacists are adept at weighing the risks versus benefits of treatment regimens. The long-term benefits of tight glycemic control need to be balanced against the harmful effects of hypoglycemia, especially in older patients and those with limited life expectancy or with severe comorbidities.71 Greater emphasis is currently being placed on outcomes, including cardiovascular events, not just on surrogate markers, such as the A1C.72 Through medication therapy management and the expanding role of the pharmacist, the clinical pharmacist is at the forefront of optimizing the care of patients with diabetes.
REFERENCES
1. Sazudek CD, Brick JC. The clinical use of hemoglobin A1c. J Diabetes Sci Technol. 2009;3(4): 629-34.
2. Hare MJL, Shaw Je, Zimmet PZ. Current controversies in the use of hemoglobinA1c. J Intern Med. 2012;271: 227-36.
3. Rhea JM, Molinaro R. Pathology consultation on HbA1c methods and interferences. Am J Clin Pathol. 2014;141(1):5-16.
4. International Expert Committee. International Expert Committee report on the role of the A1c essay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
5. American Diabetes Association. Executive summary: standards of medical care in diabetes- 2010. Diabetes Care. 2010;33(suppl 1):S4-S6.
6. World Health Organization. Use of glycated hemoglobin (Hba1c) in the diagnosis of diabetes mellitus. Geneva: World Health Organization, 2011. www.who.int/diabetes/publications/report-hba1c_2011.pdf. Accessed July 10, 2018.
7. The Diabetes Control and Complications Trial Research Group. The effective of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
8. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. (UKPDS 33). Lancet. 1998;352:837-853.
9. National Glycohemoglobin Standardization Program. www.ngsp.org. Accessed July 14, 2018.
10. National Glycohemoglobin Standardization Program. International Federation of Clinical Chemistry (IFCC) Standardization of HbA1c. www.ngsp.org/docs/ifccstd.pdf Accessed 7/14/18.
11. Nathan DM, Kuenen J, Borg R, et al for the A1c-Derived Average Glucose Study Group. Translating the A1c assay into estimated average glucose values. Diabetes Care. 2008;31:1473-8.
12. American Diabetes Association. Chapter 6. Glycemic targets: standards of medical care in diabetes- 2018. Diabetes Care. 2018;41(suppl 1):S55-S64.
13. Little RR, Rohlfing C. The long and winding road to optimal HbA1c measurement. Clin Chim Acta. 2013;418:63-71.
14. National Glycohemoglobin Standardization Program. HbA1c and estimated average glucose. www.ngsp.org/A1ceAG.asp. Accessed July 14, 2018.
15. American Diabetes Association. American Diabetes Association standards of medical care in diabetes-2018. 2018;41(suppl 1:S1-S159. http://care.diabetesjournals.org/content/diacare/suppl/2017/12/08/41.Supplement_1.DC1/DC_41_S1_Combined.pdf. Accessed July 10, 2018.
16. Garber AJ, Abrahamson MJ, Barzilay JI, et al Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summery. Endo Pract. 2018;24:91-120. www.aace.com/sites/all/files/diabetes-algorithm-executive-summary.pdf. Accessed July 10, 2018.
17. Qaseem A, Wilt TJ, Kansagara D, et al for the Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-76. http://annals.org/aim/fullarticle/2674121/hemoglobin-1c-targets-glycemic-control-pharmacologic-therapy-nonpregnant-adults-type Accessed July 10, 2018.
18. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes–2018. Ch 2. Diabetes Care. 2018;41(suppl 1):S13-S27.
19. Unnikrishnan R, Anjana RM, Mohan V. Drugs affecting HbA1c levels. Indian J Endocrinol Metab. 2012;16(4):528-531.
20. Radin MS. Pitfalls in hemoglobin A1c measurement when results may be misleading. J Gen Intern Med. 2013;29:388-394.
21. Weykamp C. HbA1c: a review of analytical and clinical aspects. Ann Lab Med. 2013;33:393-400.
22. National Glycohemoglobin Standardization Program (NGSP). Factors that interfere with HbA1c test results. www.ngsp.org/factors.asp. Accessed July 14, 2018.
23. Wang P. What clinical laboratorians should do in response to extremely low hemoglobin A1c results. Lab Med. 2016;48:89-92.
24. Sacks DB. A1c versus glucose testing: a comparison. Diabetes Care. 2011;34:518-523.
25. Bazerbachi F, Nazarian S, Alraiyes AH, et al. One-minute consult: is hemoglobin A1c an accurate measure of glycemic control in all diabetic patients? Cleveland Clin J Med. 2014;81(3):146-149.
26. Albright ES, Ovalle F, Bell DSH. Artifactually low hemoglobin A1c caused by use of dapsone. Endocrine Pract. 2002;8:370-372.
27. Mitchell K, Mukhopadhyay B. Drug-induced falsely low A1c: report of a case series from a diabetes clinic. Clin Diabetes. 2018;36:80-84.
28. Lai YC, Wang CS, Wang YC, et al. Falsely decreased HbA1c in a type 2 diabetic patient treated with dapsone. J Formosan Med Assoc. 2012;111:109-112.
29. Froud T, Faradji RN, Monroy K, et al. Dapsone-induced artifactual A1 reduction in islet transplant recipients. Transplantation. 2007;83:824.
30. Khan H, Nawaz M. Low HBA1c; is it dapsone? J Ayub Med Coll Abbottabad. 2018;30(2):301-303.
31. Pilla SJ, Quan AQ, Germain-Lee EL, et al. Immune-modulating therapy for rheumatologic disease: implications for patients with diabetes. Cur Diab Rep. 2016;16(10):91. Doi: 10.1007/s11892-016-0792-9. Accessed July 14, 2018.
32. Taminmi W, AljasserS, Kanan R, et al. Effects of hemoglobin S variants on the measurement of glycosylated hemoglobin A1c by four analytical methods. Int J Diabetes Dev Ctries. 2015;35:392-399.
33. Ziemer DC, Kolm P, Weintraub WS, et al. Glucose-independent, black-white differences in hemoglobin A1c levels. A cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152:770-777.
34. Kositsawat J, Freeman VL, Gerber BS, et al. Association of A1c levels with Vitamin D status in U.S. adults. Data from the National Health and Nutrition Examination Survey. Diabetes Care. 2010;33:1236-1238.
35. Kositsawat J, Kuchel GA, Tooze JA, et al for the Health ABC. Vitamin D insufficiency and abnormal hemoglobin A1c in black and white older persons. J Gerontol Med Sci. 2015;70(4):525-531.
36. Choi SW, Kweon SS, Lee YH, et al. 25-hydroxyvitamin D and parathyroid hormone levels are independently associated with hemoglobin A1c level of Korean type 2 diabetic patients: the Dong-Gu Study. PLoS One. 2016;11(6): e0158764. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0158764. Accessed July 14, 2018.
37. Lodh M, Mukhopadhyay R, Kumar B. A case of inappropriately high glycated hemoglobin. Ind J Clin Biochem. 2015;30:234-237.
38. Anantarapu S, Vaikkakara S, Sachian A, et al. Effects of thyroid hormone replacement on glycated hemoglobin levels in non-diabetic subjects with overt hypothyroidism. Arch Endocrinol Metab. 2015;59:495-500.
39. Bhattacharjee R, Thukral A, Chakraborty PP, et al. Effects of thyroid status on glycated hemoglobin. Indian Journal of Endocrinology and Metabolism. 2017;21(1):26-30.
40. National Glycohemoglobin Standardization Program (NGSP). HbA1c assay interferences. http://ngsp.org/interf.asp. Accessed July 14, 2018.
41. Rohlfing C, Hanson S, Weykamp C, et al. Effects of hemoglobin C, D, E and S traits on measurements of hemoglobin A1c by twelve methods. Clin Chem Acta. 2016;455:80-83.
42. Wheeler E, Leong A, Liu CT, et al. Impact of common genetic determinants of hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: a transethnic genome-wide meta-analysis. PLoS Med. 2017;14(9):e1002383. http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002383. Accessed July 10, 2018.
43. Gupta S, Jain U, Chauhan N. Laboratory diagnosis of HbA1c: a review. J Nanomed Res. 2017;5(4):00120. Accessed July 10, 2018.
44. Devices @ FDA. www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm Accessed July 14, 2018.
45. Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol. 2009;3:446-451.
46. Menke A, Rust KF, Savage PJ, et al. Hemoglobin A1c, fasting plasma glucose, and 2-hour plasma glucose distribution in US population subgroups: NHANES 2005-10. Ann Epidemiol. 2014;24(2):83-89.
47. Cavagnolli G, Pimentel AL, Freitas PAC, et al. Effect of ethnicity on HbA1c levels in individuals without diabetes: systematic review and meta-analysis. PLoS One. 2017;12(2):e0171315. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0171315. Accessed July 10, 2018.
48. American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes–2018. Ch. 8. Diabetes Care. 2018;41(suppl 1):S73-S85.
49. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. December 2, 2015. www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-1837338615493. Accessed July 14, 2018.
50. Redmon B, Caccamo D, Flavin P, et al for the Institute for Clinical Systems Improvement. Diagnosis and management of type 2 diabetes mellitus in adults. 16th ed. Bloomington, MN: Institute for Clinical Systems Improvement; July 2014. www.icsi.org/_asset/3rrm36/Diabetes-Interactive0412.pdf. Accessed July 22, 2018.
51. Handelsman Y, Bloomgarden ZT, Grunberger G, et al for the American Association of Clinical Endocrinologists and American College of Endocrinology–clinical practice guidelines for developing a diabetes mellitus comprehensive care plan- 2015. Endocr Pract. 2015;21(suppl 1):1-87. www.ncbi.nlm.nih.gov/pmc/articles/PMC4959114/pdf/nihms-803042.pdf. Accessed July 10, 2018.
52. American Diabetes Association. Standards of medical care in diabetes–2017. Diabetes Care. 2017;40(suppl 1):S48-S56. http://care.diabetesjournals.org/content/40/Supplement_1/S48. Accessed July 22, 2018.
53. Scottish Intercollegiate Guidelines Network. Management of diabetes: a national clinical guideline. SIGN Publication no. 116. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network. 2013. www.sign.ac.uk/assets/sign116.pdf. Accessed July 22, 2018.
54. The Management of Type 2 Diabetes Mellitus in Primary Care Work Group. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. Version 5.0. April 2017. www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf. Accessed July 22, 2018.
55. Endocrine News. Endocrine Society, ADA, AACE, AADE strongly disagree with ACP’s recent diabetes statement. March 2018. https://endocrinenews.endocrine.org/endocrine-society-ada-aace-aade-strongly-disagree-acps-recent-diabetes-statement/. Accessed July 24, 2018.
56. AACE Online Newsroom. The American Association of Clinical Endocrinologists, the American Diabetes Association®, the American Association of Diabetes Educators and the Endocrine Society Strongly Disagree with the American College of Physicians’ Guidance for Higher Blood…March 9, 2018. https://media.aace.com/press-release/american-association-clinical-endocrinologists-american-diabetes-association-american. Accessed July 24, 2018.
57. Joslin Diabetes Center. Joslin Diabetes Center strongly disagrees with American College of Physicians’ newly released guidance for physicians recommending higher A1c targets for non-pregnant adults with type2 diabetes. www.joslin.org/news/disagreement-with-ACP-new-guidelines-for-higher-a1c-targets.html. Accessed July 24, 2018.
58. Ismail-Beingl F, Craven T, Banerji MA, et al for the ACCORD trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430.
59. Patel A, MacMahon S, Chalmers J, et al for the ADVANCE Investigators. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
60. Duckworth W, Abraira C, Moritz T, et al for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139.
61. Gabbay RA. Guidance that allows for higher A1c misses the mark. www.ajmc.com/journals/evidence-based-diabetes-management/2018/march-2018/guidance-that-allows-for-higher-a1c-misses-the-mark. Accessed July 24, 2018.
62. Busko M. HbA1c below 8% in diabetes for ‘most’ says ACP, but others disagree. Medscape. www.medscape.com/viewarticle/893421. Accessed July 24, 2018.
63. National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes blood tests for people of African, Mediterranean, or Southeast Asian descent. www.niddk.nih.gov/health-information/diagnostic-tests/diabetes-blood-tests-african-mediterranean-southeast-asian. Accessed July 24, 2018.
64. National Glycohemoglobin Standardization Program (NGSP). Clinical use. www.ngsp.org/ada.asp. Accessed July 24, 2018.
65. Little RR. Usefulness of glycated albumin assay for diabetes monitoring. J Diabetes Sci Technol. 2011;5:1463-1465.
66. Sofronescu AG, Williams LM, Andrews DM, et al. Clin Chem. 2011;57:153-157.
67. Cranor CW, Bunting BA, Christensen DB. The Asheville Project: long-term clinical and economic outcomes in a community pharmacy diabetes care program. J Am Pharm Assoc. 2003;43:173-184.
68. Fazel MT, Bagalagel A, Lee JK, et al. Impact of diabetes care by pharmacists as part of health care team in ambulatory settings: a systematic review and meta-analysis. Ann Pharmacother. 2017;51:890-907.
69. Pousinho S, Morgado M, Falcao A, et al. Pharmacist interventions in the management of type 2 diabetes mellitus: a systematic review of randomized controlled trials. J Manag Care Spec Pharm. 2016;22:493-515.
70. Campbell RK. Role of the pharmacist in diabetes management. Am J Health-system Pharm. 2002;59(suppl 9):S18-S21.
71. Riddle MC, Gerstein HC, Holman RR, et al. A1c targets should be personalized to maximize benefit while limiting risks. Diabetes Care. 2018;41:1121-1124.
72. Lipska KJ, Krumholz HM. Is hemoglobin A1c the right outcome for studies of diabetes? JAMA. 2017;317:1017-1018.
For additional resources on A1C testing, please visit the NGSP’s links page at www.ngsp.org/links.asp.





