US Pharm. 2019:44(12):25-31.
ABSTRACT: Proton-pump inhibitors (PPIs) are commonly prescribed to patients or used for self-treatment for a variety of gastrointestinal complaints. While they are very effective, their use does not come without risk, especially when taken long-term. The pharmacist plays an important role in the judicious use of PPIs, particularly in educating patients on appropriate use to maximize their effectiveness and assisting with deprescribing PPIs, when possible.
More than 25% of the population are affected by acid-related conditions, such as dyspepsia or gastroesophageal reflux disease (GERD).1 A high number of these individuals turn to and depend upon pharmacologic options for symptomatic relief and disease-state management. Since their introduction in 1989, proton-pump inhibitors (PPIs) have become one of the most commonly utilized medications worldwide, accounting for over $11 billion in expenditures annually.2,3 In the United States alone, it is estimated that over 100 million PPIs have been prescribed per year for the past 5 years.4 Currently, PPIs remain one of the top-selling drug classes in the country.5
An estimated 113 million PPI prescriptions are filled globally every year.5 With their various indications, efficacy, and applicability for use in both ambulatory and inpatient settings, as well as with their availability as both OTC and prescription drugs, it is easy to see why PPIs have become a mainstay of pharmacotherapy for the management of acid-related disorders. Their versatility and profound use have ultimately led to their inclusion on the World Health Organization list of essential medications.6
There are currently six FDA-approved PPIs available on the market. Given their clinical effectiveness and cost, they remain a top choice among patients and providers as a therapy option for a multitude of indications.7
Indications
Because of their exceptional efficacy and long-believed tolerability and safety, the resultant exponential increase in usage has ultimately led and contributed to their inappropriate and growing overuse.7 This had led the FDA to release numerous safety statements and publish recommendations for PPI use for the following areas only, either as short-term use or long-term therapy. (See TABLE 1 for FDA-approved indications and doses for PPI therapy.2)
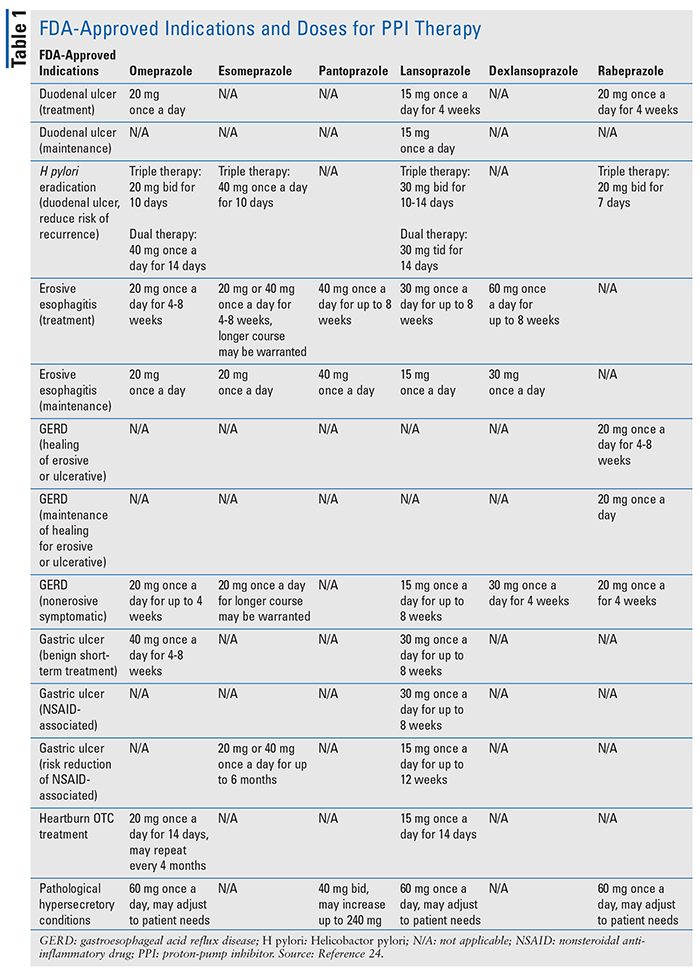
Peptic Ulcer Disease
The term peptic ulcer disease (PUD) refers to peptic acid injury of the digestive tract, primarily in the stomach and duodenum, that results in mucosal breakage reaching the submucosa.8 This can be further categorized into a gastric or duodenal ulcer based upon the location. Traditionally believed to be caused from stress and/or dietary factors, the discovery of Helicobactor pylori and studies regarding the use of nonsteroidal anti-inflammatory drugs (NSAIDs) have shifted this ideology, and these are currently cited as the main risk factors for the development of PUD.9
PPIs are FDA-approved and indicated for the eradication of H pylori (when used with antibiotics). PPIs in combination with amoxicillin and clarithromycin for 7 to 14 days (termed PPI-based triple therapy) have historically been the standard first-line therapy option for the treatment of PUD associated with H pylori infection. However, as the prevalence of antibiotic resistance has increased over the years, treatment regimens have altered to include alternative agents such as bismuth subsalicylate, metronidazole, and various others. Still, PPIs remain the base of all of the various combinations and therapy modalities available.9
NSAID-induced gastroduodenal ulcers are estimated to account for thousands of gastrointestinal (GI) complications each year, including GI bleeds, gastric pain, or even death.8 Currently, PPIs are recommended and FDA-approved as chronic prophylaxis in individuals with high risk due to concurrent and planned long-term NSAID use, as well as acutely for the treatment and healing of active ulcers, with most cases resolving with 6 to 8 weeks of therapy.9,10
GERD
GERD is one of the most prevalent acid-related disorders that require acid-suppressive therapy. PPIs have become the drug of choice for numerous patients and providers due to efficacy in symptomatic relief of GERD and other nonerosive reflux diseases. However, due to the underlying pathophysiological mechanisms responsible for GERD, PPI use typically requires chronic use and is not a curative therapy but may also be taken on an as-needed basis for the management of symptomatic flares.11
GERD also has the potential to develop or manifest into other conditions that require chronic PPI use. These include disorders such as Barrett’s esophagus or esophageal strictures. In addition, erosive esophagitis may develop, in which PPIs remain an appropriate option for both the healing and maintenance of such an event.11,12 The FDA does approve the use of PPIs in these aforementioned areas.
Zollinger-Ellison Syndrome and Pathological Hypersecretory Conditions
Zollinger-Ellison syndrome (ZES) is an acid hypersecretory condition caused by a gastrin-secreting tumor.12 PPIs are the FDA-approved drug of choice for management and must be given chronically to control acid secretion and prevent or reduce complications and symptoms in most patients with ZES.12
Stress Ulcer Prophylaxis
Although not FDA-approved, numerous guidelines recommend PPI use as prophylaxis therapy in hospitalized patients. Stress ulcers may occur in patients admitted to intensive-care units (ICUs), and inappropriate management or prophylaxis treatment may lead to severe events such as GI bleeding or ulcer formation.11 Events such as GI bleeds may occur in up to 15% of patients not on stress ulcer prophylaxis (SUP).11,13 Although SUP is critical to improve hospitalized patient outcomes, it should be stressed that PPIs are only approved for SUP in high-risk patients, defined as those who are critically ill and on mechanical ventilation for more than 48 hours, or those on anticoagulation.11 PPI use in these patients should be limited to short-term therapy as appropriate. PPIs should not be used as prophylaxis in low-risk or noncritically ill hospitalized patients.
Other Indications
PPIs are commonly used for a variety of other indications that do not carry an FDA approval. These include as add-on therapy for patients on antiplatelet therapy with high risk of GI bleed; functional dyspepsia; and prior to or following an endoscopy associated with an acute or high risk of bleeding.7
Adverse Events of PPIs
As PPI use continues to grow every year, concerns regarding their safety have arisen. Throughout the years, the FDA has published numerous safety statements and recommendations with regards to appropriate PPI use as new data have become available. Although once thought of as safe options, PPI usage in recent years has raised concerns, evidenced by numerous studies and reviews exploring a diverse range of adverse outcomes associated with PPI use. (See TABLE 2 for a list of PPI-associated adverse events and mechanisms.)
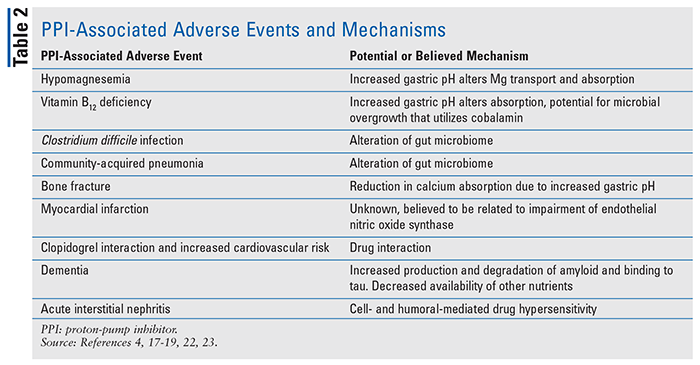
Hypomagnesemia
In 2011, the FDA issued a warning that long-term PPI use may lower serum magnesium levels that supplementation alone may not correct unless the PPI was discontinued.14 When severe, hypomagnesemia may present in the form of muscle weakness, tetany, seizures, cardiac arrhythmias, and hypotension, with the potential to be life-threatening.15 The risk of hypomagnesemia was further studied by a 2015 systematic review and meta-analysis that included over 100,000 patients and assessed the risk of hypomagnesemia in patients with PPI as compared with non-PPI users. This study ultimately concluded approximately a 40% increased risk of hypomagnesemia with PPI use as compared with non-PPI therapy.4
Infections
In 2015, the FDA issued a public safety alert in regards to increased Clostridium difficile infections associated with PPI use. This was primarily based upon a 2012 systematic review and meta-analysis study that included over 30 studies and 300,000 patients, which concluded that PPI users had a 74% higher risk of developing a C diff infection, as well as a 2.5-fold higher risk of recurrent infections, as compared with nonusers.16
In addition to increased C diff infections, PPI use may also lead to increased rates of pneumonia. A meta-analysis performed in 2011 showed that the risk of community-acquired pneumonia (CAP) was 34% higher in patients on PPIs, which increased with higher dosing.17 It should be mentioned that this study did not find an increased risk with hospital-acquired pneumonia.
For both C diff and CAP, it is generally hypothesized to be due to decreased gastric acidity caused by long-term PPI use and a subsequent increase in bacterial colonization.17,18
Fractures
Osteoporosis is one of the most common bone-related disorders, with complications that are associated with high morbidity, mortality, and healthcare-associated costs.19 In May of 2010, the FDA issued a public safety statement alert regarding potential increased risk of fractures associated with PPI use.20 Since then, numerous studies exploring the relationship between PPI use and fracture risk have been examined. A 2016 meta-analysis reviewing over 200,000 fracture cases reported a 26% higher risk of hip fracture, 58% higher risk of spine fracture, and a 33% risk of fracture at any site in individuals who used PPI as compared with those who have not, even at a duration of less than 1 year.19
Cardiovascular Disease
Individuals with acute coronary syndrome (ACS) who have undergone percutaneous coronary intervention are commonly prescribed antiplatelet therapy to reduce and prevent further cardiovascular complications. PPIs may be prescribed in conjunction with antiplatelet therapy to reduce risk of gastrointestinal bleeding. In addition, as mentioned, PPIs are used commonly by the general populace as a whole for other acid-related disorders.24 In 2009, the FDA issued a statement warning against the combination of the antiplatelet agent clopidogrel and PPIs due potential drug interactions between the two.21 Mechanistically, clopidogrel is metabolized to its active form through the same liver enzymes that metabolize PPIs, raising concern of a potential diminished antiplatelet effect and therefore an increase in cardiovascular events.15
Currently, data surrounding the clinical significance of this event are mixed. A 2015 meta-analysis of 31 observational studies found that individuals on PPI therapy and clopidogrel had a 30% increased risk of cardiovascular events as compared to nonusers of PPI therapy.22 However, the four randomized clinical trials included in the review found there was no increased risk of events identified.22
In addition, another 2015 systematic review that explored the use of PPIs and the risk of myocardial infarction found a 16% increased risk associated with use. This was found to be regardless of clopidogrel use and was not seen with H2 blocker therapy.5
Other Concerns
There are numerous other concerns associated with PPI use for which the FDA has not released statements. These include issues such as subacute cutaneous lupus, dementia, acute interstitial nephritis, and vitamin B12 deficiency.23 Overall, the safety of PPIs remains controversial. As numerous new reports and literature are published, the use of PPIs and safety of PPI therapy should be recommended with hesitancy over other options.
Deprescribing PPIs
In 2017, guidelines for deprescribing PPIs were published in Canadian Family Physician. A team of healthcare professionals, including three pharmacists, collaborated to establish the evidence-based clinical practice guideline.25 Deprescribing is defined as reducing the dose, stopping, or using “on-demand” dosing. The guideline recommends deprescribing PPIs in adults who suffer from heartburn and who have completed a minimum treatment of 4 weeks in which symptoms are relieved. These recommendations do not apply to patients with Barrett’s esophagus, severe esophagitis, or patients with a history of bleeding gastrointestinal ulcers.8 Per the published guidelines, an algorithm can be used in determining when and how PPIs should safely be deprescribed. For patients needing occasional symptom relief, OTC antacids or H2 receptor antagonists (H2RA) may be used on an as-needed basis. H2RAs may be used on a daily basis, although the recommendation only proves to have moderate-quality evidence.25 Patients should also be educated on the nonpharmacologic approaches to minimize symptoms of heartburn, dyspepsia, regurgitation, and epigastric pain. Patients should be counseled to avoid meals 2 to 3 hours before bedtime, avoid dietary triggers, and address whether weight loss is required.25
Pharmacists’ Role in Judicious PPI Use
As members of the healthcare team, pharmacists have a vital role in educating patients on the proper use of PPIs. Approximately 50% of patients taking PPIs for nonerosive GERD still experience unresolved symptoms.8 When symptoms persist despite the use of once-daily dosing, the American Gastroenterology Association and the American College of Gastroenterology practice guidelines recommend increasing to twice-daily dosing. Increasing the dose may be necessary for some, but it is not always the best course of action. PPIs require active proton pumps to establish binding and, therefore, make it essential for preprandial administration. Nearly half of patients taking PPIs do not take their dose before eating, thus leading to decreased binding and efficacy.8 In order to reduce PPI use, stewardship programs, managed by pharmacists, have been established to determine if use is necessary, educate patients on the proper administration, and discuss whether deprescribing is warranted.
Involving patients in the decision to deprescribe PPIs is vital for patient success. Patients who are educated on the risks associated with long-term therapy and possible side effects associated with PPIs are more likely to understand the reasoning for deprescribing and may experience better long-term outcomes.25
REFERENCES
1. Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157(3):682-691.
2. Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton pump inhibitors: review of emerging concerns. Mayo Clin Proc. 2018;93(2):240-246.
3. Lehault WB, Hughes DM. Review of the long-term effects of proton pump inhibitors. Federal Practitioner. 2017;34(2):19-23.
4. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241.
5. Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One. 2015;10(6):e0124653.
6. Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017;153(1):35-48.
7. Yadlapati R, Kahrilas PJ. When is proton pump inhibitor use appropriate? BMC Med. 2017;15(1):36.
8. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27-37.
9. Lanas A, Chan FK. Peptic ulcer disease. The Lancet. 2017;390(10094):613-624.
10. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am Gastroenterol. 2009:104(3):728-738.
11. Savarino V, Dulbecco P, de Bortoli N, et al The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
12. Spechler SJ. Barrett’s esophagus. In Shaker R, Belafsky P, Postma G, Easterling C, eds. Principles of Deglutition. New York, NY: Springer; 2013.
13. Madsen KR, Lorentzen K, Clausen N, et al. Guideline for stress ulcer prophylaxis in the intensive care unit. Dan Med J. 2014;61(3):C4811.
14. U.S. Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs). 2011. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-low-magnesium-levels-can-be-associated-long-term-use-proton-pump.
15. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med. 2016;176(2):172-174.
16. Kwok CS, Arthur AK, Anibueze CI, et al. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019.
17. Eom CS, Jeon CY, Lim JW, et al. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. Canadian Medical Association Journal. 2011;183(3):
310-319.
18. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;175(5):784-791.
19. Zhou B, Huang Y, Li H, et al. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int. 2016;27(1):339-347.
20. US Food and Drug Administration. FDA drug safety communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. Postmarket Drug Safety Information for Patients and Providers. 2011. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-possible-increased-risk-fractures-hip-wrist-and-spine-use-proton-pump.
21. Focks JJ, Brouwer MA, van Oijen MG, et al. Concomitant use of clopidogrel and proton pump inhibitors: impact on platelet function and clinical outcome-a systematic review. Heart. 2013;99(8):520-527.
22. Melloni C, Washam JB, Jones WS, et al. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review. Circ Cardiovasc Qual Outcomes. 2015;8(1):47-55.
23. Ambizas EM, Etzel JV. Proton pump inhibitors: considerations with long-term use. US Pharm. 2017;42(7):4-7.
24. Centers for Medicare and Medicaid Services. Proton pump inhibitors: Food and Drug Administration-approved indications and dosages for use in adults. Department of Health and Human Services. 2013. www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Pharmacy-Education-Materials/Downloads/ppi-adult-dosingchart11-14.pdf.
25. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors evidence-based clinical practice guideline. Can Fam Physician. 2017;63:354-364.
To comment on this article, contact rdavidson@uspharmacist.com.





