US Pharm. 2021;46(10):60-CV3.
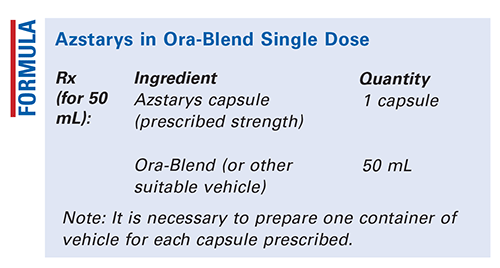
Method of Preparation: Accurately obtain and/or measure each ingredient.1 Each ingredient is to be dispensed separately. For administration, the patient should empty the contents of a single capsule into the bottle of Ora-Blend (or other suitable vehicle).2 The patient should shake it well until thoroughly mixed and take entire contents at one time. Once combined, the mixture should not be stored for later use.
Use: Azstarys is a new central nervous system (CNS) stimulant that may be used in the treatment of attention-deficit/hyperactivity disorder in patients aged 6 years and older.3
Packaging: Package in separate tight, light-resistant containers.1
Labeling: Keep out of reach of children. Empty the contents of the single capsule into the bottle of liquid and shake well until thoroughly mixed. Take the entire contents at one time. The mixture, once combined, should not be stored for later use.
Stability: Once mixed, this preparation should be taken immediately or within 10 minutes of mixing. Do not store after mixing.3
Discussion: The labeling for Azstarys states that patients who cannot swallow the capsules should open the capsule and sprinkle the entire contents onto 2 tablespoons of applesauce or into 50 mL of water.3 For those patients who find either of these options unacceptable or who find the taste disagreeable, the formula presented here should provide a reasonable alternative. Different vehicles, according to the preference of the patient, could also be used.
Azstarys (serdexmethylphenidate and dexmethylphenidate) capsules contain dexmethylphenidate, a CNS stimulant, and serdexmethylphenidate, a prodrug of dexmethylphenidate. Azstarys capsules are intended for oral administration, and each capsule contains a fixed molar ratio of 30% dexmethylphenidate and 70% serdexmethylphenidate. Azstarys contains 26.1/5.2, 39.2/7.8, or 52.3/10.4 mg of serdexmethylphenidate/dexmethylphenidate (equivalent to 28/6, 42/9, or 56/12 mg of serdexmethylphenidate chloride/dexmethylphenidate hydrochloride, respectively). The combined molar dose of serdexmethylphenidate and dexmethylphenidate in each dosage strength of Azstarys is equivalent to 20, 30, or 40 mg dexmethylphenidate hydrochloride, respectively (equivalent to 17.3, 25.9, or 34.6 mg dexmethylphenidate free base, respectively).3
Serdexmethylphenidate (C25H30N3O8Cl, MW 535.98) is a white to off-white, crystalline powder. Its solutions are acid to litmus. It is freely soluble in water and slightly soluble in alcohol.3
Dexmethylphenidate hydrochloride (C14H19NO2.HCl, MW 269.77) is a white to off-white powder. Its solutions are acid to litmus. It is freely soluble in water and soluble in alcohol.3
Inactive ingredients in the capsules include colloidal silicon dioxide, crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, and talc. Each strength also contains colorants in the capsule shell as follows: 26.1/5.2 mg—black iron oxide, FD&C Blue No. 1, and titanium dioxide; 39.2/7.8 mg—black iron oxide, FD&C Blue No. 1, FD&C Red No. 40, and titanium dioxide; 52.3/10.4 mg—black iron oxide, FD&C Red No. 40, FD&C Yellow No. 6, and titanium dioxide.3
In pediatric patients aged 6 to 12 years, the recommended starting dosage of Azstarys is 39.2 mg serdexmethylphenidate/7.8 mg dexmethylphenidate once daily in the morning. After 1 week, this may be either increased to a dosage of 52.3 mg serdexmethylphenidate/10.4 mg dexmethylphenidate per day or decreased to a dosage of 26.1 mg serdexmethylphenidate/5.2 mg dexmethylphenidate per day, depending on response and tolerability.3
In adults and in pediatric patients aged 13 to 17 years, the recommended starting dosage of Azstarys is 39.2 mg serdexmethylphenidate/7.8 mg dexmethylphenidate once daily in the morning. After 1 week, increase the dosage to 52.3 mg serdexmethylphenidate/10.4 mg dexmethylphenidate per day.3
Azstarys is a federally controlled substance (CII) because it can be abused or lead to dependence; therefore, any remains should be disposed of in compliance with local laws and regulations on drug disposal of CNS stimulants. Dispose of remaining, unused, or expired Azstarys through a medicine take-back program or an authorized collector registered with the Drug Enforcement Administration. If no take-back program or authorized collector is available, mix the Azstarys with an undesirable, nontoxic substance in order to make it less appealing to children and pets; place the mixture in a container such as a sealed plastic bag; and dispose of the container in the household trash.
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; September 2021.
2. Ora-Blend product information. Allegan, MI: Perrigo; 2021.
3. Azstarys (serdexmethylphenidate and dexmethylphenidate) package insert. Grand Rapids, MI: Corium, Inc; June 2021.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






