US Pharm. 2008;33(6):HS-3-HS-9.
Polyomavirus infection is an
emerging challenge wherein nephritis and graft loss may affect up to 10% of
kidney-transplant recipients.1 Two types of polyomaviruses in the
Polyomaviridae family, BK and JC, appear to predominantly cause clinical
disease in immunocompromised hosts. These are small, nonenveloped DNA viruses;
however, they vary in their clinical manifestations of viral nephritis and
viral encephalopathy, respectively. Primary infection with BK virus (BKV) is
not well known, but is generally asymptomatic; it may be transmitted through
ingesting contaminated water or food, via the respiratory system,
transplacentally, or from donor tissue.2-4 The virus remains latent
in the renal epithelium and is activated during the immunocompromised state as
a result of transplantation or increased immunosuppression. It may occur when
the donor or recipient's tissue is infected. Donors with higher BKV titers
have been shown to have an increased risk of causing BKV infection in
recipients versus donors with lower titers; recipients who had BKV infection
and received a kidney from the same donor have been shown to have identical
BKV genotypes.3,5,6
BKV was first recognized in a
renal-transplant patient diagnosed with the disease in 1971 as a result of
virus reactivation due to new potent immunosuppressive medications aimed at
reducing acute rejection and improving allograft survival.7
BKV-induced interstitial nephritis (BK nephropathy) is not directly caused by
one specific medication, but it has been reported in studies with drug
regimens that include more intense immunosuppression with tacrolimus (TAC),
mycophenolate mofetil (MMF), or the combination.8-11 Reducing
immunosuppression is the cornerstone of management, but the risk of
predisposing patients to acute and chronic rejections must be considered. No
approved antiviral medication is available, but leflunomide, cidofovir, IV
immunoglobulin (IVIG), and fluoroquinolones have been used.1,9
Clinical Risk Factors
BK nephropathy
remains poorly understood despite its increasing incidence. Potent
immunosuppression is believed to be the cause of the resurgence of BKV in
kidney-transplant patients. Factors that may be associated with the risk of BK
nephropathy include older age, male gender, white ethnicity, diabetes,
renal-tissue injury from ischemia, presence of cytomegalovirus (CMV), acute
rejection, and treatment with high-dose steroid pulses.1,9 There
have been 10 cases in which the absence of HLA-C7 allele (a genetic trait) was
observed in donors and recipients with sustained BK viremia.3 The
precise role of the HLA-C7 gene is unknown, but its absence may
increase the risk that infection will advance to sustained viremia, a
preceding factor in BK nephropathy.
Clinical Features
The clinical
manifestation of BK nephropathy varies from the asymptomatic state of viremia
to hematuria, ureteral stenosis, and interstitial nephritis. BK nephropathy
closely simulates acute rejection in multiple reports where the onset of
disease occurs at an average period of 10 to 13 months posttransplantation;
however, onset of nephritis may occur early (six days posttransplantation) or
be delayed (five years).9,12-14 The first sign of BK nephritis is
renal dysfunction (serum creatinine [SCr] >2.2 mg/dL), which correlates to a
decline in long-term graft survival that may be accompanied by fever and a
urinalysis consisting of decoy cells reflecting the disease state.8,12
Recently, implemented routine surveillance measures such as posttransplant
protocol biopsy also have detected BK nephritis in the absence of SCr
elevation.15,16
Diagnosis
Viral replication
begins early after transplantation, and studies have suggested that BK viral
infections progress through detectable stages: viruria, then viremia, and
finally nephropathy.17-20 Findings suggestive of interstitial
nephritis in patients lead to a clinical suspicion of BKV infection, prompting
laboratory tests to detect the presence of BKV. Although various laboratory
tests exist (TABLE 1), the 2005 Consensus Conference states that a
definitive diagnosis is established by renal-allograft biopsy.1,21
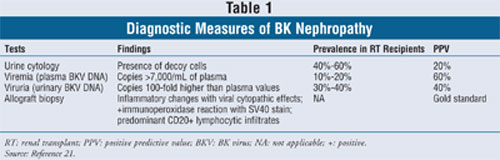
Urine cytology remains the
most common method to monitor and detect the presence of BKV after
transplantation. It is a simple and inexpensive test for identifying patients
at risk since asymptomatic shedding or urinary decoy cells usually foreshadow
the development of BK nephropathy. However, acute cellular rejection, TAC
nephrotoxicity, and acute tubular necrosis also may result in transient
shedding of virus-infected cells.21 Thus, the elimination of decoy
cells in the urine suggests active BKV infection but does not always confirm
BK nephropathy. Patients demonstrating persistent viral cytopathic effects
will need further tests.
Plasma polymerase chain
reaction (PCR) studies to detect BKV DNA are more reliable as a clinical tool.
This is because viremia (detectable virus in the plasma) is another clinical
manifestation of BK infection that correlates closely with allograft
involvement, where it is seen in nearly 100% of cases of BK nephropathy.
22-24 Formulating an early diagnosis, determining clinical response to
antiviral therapy, and monitoring for relapse are some of the advantages of
utilizing PCR. Alternatively, evaluation of BKV DNA in the urine via
quantitative PCR seems to be a viable detection method since the BKV load is
100-fold to 1,000-fold higher in urine than in plasma.24 Urine BKV
PCR, however, is a relatively expensive test without clear benefits over
plasma BKV PCR.
Renal-allograft biopsy remains
the gold standard for diagnosing BK nephropathy. BK nephropathy may be missed
in one-third of biopsies due to the focal nature of early BK nephropathy;
examination should therefore include two core biopsies, preferably of the
medulla, where the virus is more likely to be present.25,26 In
cases where initial biopsy does not confirm BK nephropathy, preemptive
treatment or repeat biopsy may be considered. Preemptive treatment should be
reserved for patients with presumptive BK nephropathy, as defined by
exhibition of characteristically high levels of BK replication (plasma BKV PCR
load >10,000 copies/mL).1,25
Treatment
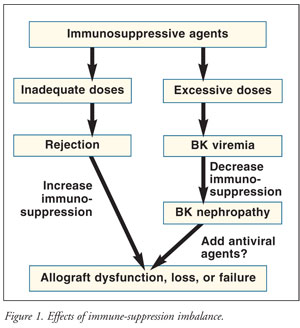
Before treatment of BK nephropathy is commenced, allograft biopsy and quantification of baseline polyomavirus load in either urine or plasma should be performed to confirm the diagnosis, with subsequent monitoring every two to four weeks. The therapeutic goal of managing BKV infection is to eliminate the virus, avoid acute rejection, and preserve renal function. The principal treatment is to maintain a balance of immune suppression; inadequate control will result in rejection, and overimmunosuppression may lead to BK nephropathy (FIGURE 1). One strategy is to discontinue the antimetabolic agent (azathioprine or MMF) and reduce the calcineurin inhibitor; another is to switch from TAC to low-dose cyclosporine. Brennan et al demonstrated that preemptive withdrawal of MMF upon detection of viremia prevented BK nephropathy without acute rejection or graft loss.27 Another study found that reducing the dose of calcineurin inhibitor, but not decreasing overall immunosuppression, was associated with recovery of renal function.12 Interestingly, studies that incorporate steroid-withdrawal maintenance protocols seem to have a decreased incidence of patients developing BK nephropathy.18,28 Since none of the therapies has proven efficacy, the cornerstone of treatment is to decrease maintenance immunosuppression. Adjuvant antiviral agents ( TABLE 2) may be considered in patients who present with progressive allograft dysfunction based upon small clinical case studies.29
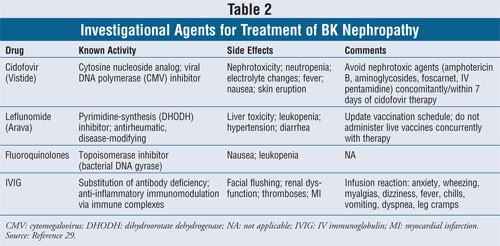
Cidofovir is approved as an IV
treatment for CMV retinitis in AIDS patients. The use of cidofovir was
initially explored in HIV-infected patients diagnosed with progressive
multifocal leukoencephalopathy, which is a fatal demyelinating disease caused
by polyomavirus infection. Limited studies exist on the use of cidofovir for
BK nephropathy, and it is not known how cidofovir exerts anti-BKV activity.
The case report by Keller et al showed BKV clearance and histologic
improvement upon repeat biopsy in a renal-transplant recipient.30
Therapeutic use of cidofovir
is limited by its nephrotoxic effects, including proteinuria and renal
failure. To reduce nephrotoxicity in BK nephropathy, cidofovir doses are five-
to 10-fold lower than those given for CMV therapy. In a retrospective study,
eight of 21 patients received adjuvant low doses of cidofovir (0.5-1.0 mg/kg
for 4-10 weekly regimens) plus reduced immunosuppressive therapy and the other
13 were treated with reduced immunosuppressive therapy alone.31 The
patients given adjuvant low-dose cidofovir therapy retained their allografts,
whereas nine of the 13 who had reduced immunosuppressive therapy alone
experienced graft loss.
Leflunomide is indicated for
the treatment of patients with active rheumatoid arthritis. It is a prodrug
with an active metabolite that has both antiviral and immunosuppressive
effects that are thought to relate to its activity in BK nephropathy, but the
exact mechanism of action remains unclear. In one report, treatment of 17
patients with leflunomide plus discontinuation of MMF and dose decrease of TAC
led to a reduction in viremia and viruria without clinically significant
adverse events.32 Patients were treated with loading doses of
leflunomide 100 mg/day for five days and maintenance doses of 20 to 60 mg/day,
with a target blood level of 50 to 100 µg/mL.32 Additionally,
a prospective, open-label study of 12 patients reported that treatment
consisting of reduced immunosuppression and leflunomide led to improved graft
function (66.6%) and cleared BKV viremia (42%); it also produced anemia and
mild thrombocytopenia (17%).33 In both studies, it is unknown
whether the decrease in viremia and viruria was caused by the use of
leflunomide or by the decreased immunosuppression.32,33
IVIG holds established
indications for a number of autoimmune disorders and primary immune
deficiencies through its immunomodulatory properties derived from macrophage
inhibition. It is used in transplant patients to treat viral infection, but
evidence of its efficacy is often lacking. IVIG has been used successfully to
treat allograft rejection, and it may be effective for polyomavirus. Since it
can be difficult to differentiate between rejection and BK nephropathy, it may
be beneficial to use IVIG based upon plasma PCR. In a recent report, eight
patients received IVIG 2 g/kg over five days in addition to a reduction in
immunosuppressive therapy.34 After a mean follow-up of 15 months,
seven patients (88%) had functional grafts, but renal function remained
impaired.34 IVIG is a costly treatment that has significant side
effects including allergic reactions, renal dysfunction, and thrombotic
events, and in rare cases it can cause aseptic meningitis.
Fluoroquinolones are
broad-spectrum antibiotics that inhibit bacterial DNA synthesis via the
inhibition of two bacterial enzymes, DNA gyrase and topoisomerase IV. These
medications may achieve high tissue concentrations, particularly in the renal
tubulus, possibly as a result of inhibition of BKV T antigenñcoded helicase
activity.29 A study of 30 hematopoietic stem-cell transplant
patients receiving ciprofloxacin 500 mg twice daily found that treatment
reduced the urinary BKV load.35 Fluoroquinolones are the
most cost-efficient of the investigational agents, but their use is limited
due to lack of data.
Screening
There are no
definitive guidelines for screening patients to prevent BK nephropathy.
Screening may help identify patients at risk for BKV replication and thus lead
to targeted reduction of immunosuppression that can resolve the infection.
Earlier diagnosis of BK nephropathy coincides with an increased success rate
for treatment and decreased morbidity and mortality. This approach is
effectively demonstrated by a study of 200 patients who were randomized to
either TAC (n = 134) or cyclosporine (n = 66) and were monitored with PCR of
blood and urine to help detect early viremia.28 Viruria was highest
with TAC/MMF (46%) and lowest with cyclosporine/MMF (13%).28 The
antimetabolite (MMF or azathioprine) was then discontinued, which led to
resolution of viremia in 95% of patients without an increased risk of acute
rejection, allograft dysfunction, or graft loss.28
An international panel
established screening recommendations for renal-transplant patients.1
A positive screening result should be confirmed within four weeks and
assessed by one of the quantitative assays (BKV DNA or RNA load in plasma or
urine). If the adjunctive test is above threshold values, then allograft
biopsy should be performed to definitively diagnose BK nephropathy. Despite
the benefits of early detection, routine BKV screening becomes cost-effective
only if the incidence of BK nephropathy exceeds 2.1% in a transplant center.
36
Conclusion
BK nephropathy
poses a significant complication for the posttransplant kidney recipient.
Although modern immunosuppressive agents have greatly benefited this
population, they have also led to increased rates of BK nephropathy. Screening
may identify BKV replication in recipients, and targeted reduction of
immunosuppression can resolve the infection; however, this method is reserved
for transplant centers with a high incidence of BK nephropathy. Successful
retransplantation has been achieved in patients with graft failure due to BK
nephropathy. In a study involving five transplant centers, all 10 patients had
good graft function with a mean SCr of 1.5 mg/dL after mean follow-up of more
than 2 years.37 Limited information exists on investigational
antiviral agents; prospective, randomized, controlled clinical trials are
needed to assess their efficacy and safety. BKV infection remains a challenge
until reliable measures of immunosuppression or novel agents that specifically
target the virus are available.
Pharmacists play an important
role in the treatment of kidney transplantation and serve as part of a
multidisciplinary team that counsels patients on all aspects of their
medications. Clinical responsibilities include addressing compliance, adverse
drug events, drug interactions, and appropriate dose adjustments.
Additionally, pharmacists may have contact with these patients in the pharmacy
and note that they are feeling unwell, a possible sign of rejection or BKV.
Pharmacists should be aware of the signs and symptoms of rejection and BKV in
order to direct the patient to seek immediate physician attention.
REFERENCES
1. Hirsch HH,
Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in
renal transplantation: interdisciplinary analyses and recommendations.
Transplantation.2005;79:1277-1286.
2. Bofill-Mas S,
Formiga-Cruz M, Clemente-Casares P, et al. Potential transmission of human
polyomaviruses through the gastrointestinal tract after exposure to virions or
viral DNA. J Virol.2001;75:10290-10299.
3. Bohl DL, Storch GA,
Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and
role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant.
2005;5:2213-2221.
4. Reploeg MD, Storch
GA, Clifford DB. Bk virus: a clinical review. Clin Infect Dis.
2001;33:191-202.
5. Andrews CA, Shah KV,
Daniel RW, et al. A serological investigation of BKV and JC virus infections
in recipients of renal allografts. J Infect Dis.1988;158:176-181.
6. Vera-Sempere FJ,
Rubio L, Felipe-Ponce V, et al. Renal donor implication in the origin of BK
infection: analysis of genomic viral subtypes. Transplant Proc.
2006;38:2378-2381.
7. Gardner SD, Field
AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine
after renal transplantation. Lancet. 1971;1:1253-1257.
8. Ramos E, Drachenberg
CB, Portocarrero M, et al. BK virus nephropathy diagnosis and treatment:
experience at the University of Maryland Renal Transplant Program. Clin
Transpl. 2002;14:143-153.
9. Ramos E, Drachenberg
CB, Papadimitriou JC, et al. Clinical course of polyoma virus nephropathy in
67 renal transplant patients. J Am Soc Nephrol. 2002;13:2145-2151.
10. Mengel M, Marwedel
M, Radermacher J, et al. Incidence of polyomavirus-nephropathy in renal
allografts: influence of modern immunosuppressive drugs. Nephrol Dial
Transplant. 2003;18:1190-1196.
11. Barri YM, Ahmad I,
Ketel BL, et al. Polyoma viral infection in renal transplantation: the role of
immunosuppressive therapy. Clin Transplant. 2001;15:240-246.
12. Vasudev B,
Hariharan S, Hussain SA, et al. BK virus nephritis: risk factors, timing, and
outcome in renal transplant recipients. Kidney Int. 2005;68:1834-1839.
13. Randhawa PS,
Finkelstein S, Scantlebury V, et al. Human polyoma virus-associated
interstitial nephritis in the allograft kidney. Transplantation.
1999;67:103-109.
14. Howell DN, Smith
SR, Butterly DW, et al. Diagnosis and management of BK polyomavirus
interstitial nephritis in renal transplant recipients. Transplantation.
1999;68:1279-1288.
15. Gloor JM, Cohen AJ,
Lager DJ, et al. Subclinical rejection in tacrolimus-treated renal transplant
recipients. Transplantation. 2002;73:1965-1968.
16. Buehrig CK, Lager
DJ, Stegall MD, et al. Influence of surveillance renal allograft biopsy on
diagnosis and prognosis of polyomavirus-associated nephropathy. Kidney Int.
2003;64:665-673.
17. Limaye AP, Jerome
KR, Kuhr CS, et al. Quantitation of BK virus load in serum for the diagnosis
of BK virus-associated nephropathy in renal transplant recipients. J Infect
Dis. 2001;183:1669-1672.
18. Bressollette-Bodin
C, Coste-Burel M, Hourmant M, et al. A prospective longitudinal study of BK
virus infection in 104 renal transplant recipients. Am J Transplant.
2005;5:1926-1933.
19. Hirsch HH, Knowles
W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication
and nephropathy in renal transplant recipients. N Engl J Med.
2002;347:488-496.
20. Nickeleit V,
Klimkait T, Binet IF, et al. Testing for polyomavirus type BK DNA in plasma to
identify renal-allograft recipients with viral nephropathy. N Engl J Med.
2000;342:1309-1315.
21. Drachenberg CB,
Papadimitriou JC, Bourquin PM, et al. Persistent excretion of decoy cells in
urine predicts BK allograft nephropathy (BKN) [abstract]. Program and
abstracts of American Transplant Congress 2003: the fourth joint American
Transplant meeting; May 30-June 4, 2003; Washington, DC. Abstract 151.
22. Hariharan S. BK
virus nephritis after renal transplantation. Kidney Int.
2006;69:655-662.
23. Viscount HB, Eid
AJ, Espy MJ, et al. Polyomavirus polymerase chain reaction as a surrogate
marker of polyomavirus-associated nephropathy. Transplantation.
2007;84:340-345.
24. Hirsch HH.
Polyomavirus BK nephropathy: a (re-)emerging complication in renal
transplantation. Am J Transplant. 2002;2:25-30.
25. Drachenberg CB,
Papadimitriou JC. Polyomavirus-associated nephropathy: update in diagnosis.
Transpl Infect Dis. 2006;8:68-75.
26. Drachenberg CB,
Papadimitriou JC, Hirsch HH, et al. Histological patterns of polyomavirus
nephropathy: correlation with graft outcome and viral load. Am J
Transplant. 2004;4:2082-2092.
27. Brennan DC, Agha I,
Bohl DL. Incidence of BK with tacrolimus versus cyclosporine and impact of
preemptive immunosuppression reduction. Am J Transplant.
2005;5:582-594.
28. Matas AJ,
Kandaswamy R, Gillingham KJ, et al. Prednisone-free maintenance
immunosuppression--a 5 year experience. Am J Transplant.
2005;5:2473-2478.
29. Rinaldo CH, Hirsch
HH. Antivirals for the treatment of polyomavirus BK replication. Expert Rev
Anti Infect Ther. 2007;5:105-115.
30. Keller LS, Peh CA,
Nolan J, et al. BK transplant nephropathy successfully treated with cidofovir.
Nephrol Dial Transplant. 2003;18:1013-1014.
31. Kuypers DR,
Vandooren AK, Lerut E, et al. Adjuvant low-dose cidofovir therapy for BK
polyomavirus interstitial nephritis in renal transplant recipients. Am J
Transplant. 2005;5:197-204.
32. Williams JW, Javaid
B, Kadambi PV, et al. Leflunomide for polyomavirus type BK nephropathy. N
Engl J Med. 2005;352:1157-1158.
33. Faguer S, Hirsch
HH, Kamar N, et al. Leflunomide treatment for polyomavirus BK-associated
nephropathy after kidney transplantation. Transpl Int. 2007;20:962-969.
34. Sener A, House AA,
Jevnikar AM, et al. Intravenous immunoglobulin as a treatment for BK virus
associated nephropathy: one-year follow-up of renal allograft recipients.
Transplantation. 2006;81:117-120.
35. Leung AY, Chan MT,
Yuen KY, et al. Ciprofloxacin decreased polyoma BK virus load in patients who
underwent allogeneic hematopoietic stem cell transplantation. Clin Infect
Dis. 2005;40:528-537.
36. Kiberd BA.
Screening to prevent polyoma virus nephropathy: a medical decision analysis.
Am J Transplant. 2005;5:2410-2415.
37. Ramos E, Vincenti
F, Lu WX, et al. Retransplantation in patients with graft loss caused by
polyoma virus nephropathy. Transplantation. 2004;77:131-133.
To comment on this article, contact
rdavidson@jobson.com.





