US Pharm. 2012;37(8):38-41.
Listeriosis is a serious, potentially fatal infection caused by Listeria species.1 Listeria species are found throughout the environment, residing in soil, water, sewage, vegetation, wild animal feces, farms, and food-processing facilities.2 Infections, which are usually the result of foodborne contamination, are generally sporadic, but the CDC has documented several multistate outbreaks. The most recent outbreak was in late 2011, when Listeria-contaminated cantaloupes from a farm in Colorado caused infection in 146 people, with symptoms ranging from febrile gastroenteritis to meningoencephalitis. The outbreak encompassed 28 states and led to 30 deaths.3 Although most cases of listeriosis are self-limiting, it is imperative to utilize appropriate antibiotic therapy in high-risk patients to prevent the occurrence of invasive listeriosis and even fatality.
Epidemiology
In the United States, L monocytogenes is estimated to cause 1,600 illnesses, more than 1,400 hospitalizations, and 250 deaths per year. Immunocompromised individuals are at greatest risk; for example, a person with AIDS is 300 times more likely than a person with normal immune function to develop infection.1 Because of pregnancy-induced immunosuppression, pregnant women are 13 times more likely to become infected.1 In fact, 27% of all reported Listeria infections are in pregnant women.4 The estimated incidence rate of neonatal infections is 8.6 per 100,000 live births.4 Case fatality for fetal or neonatal infections ranges from 20% to 30%.4 Even after diagnosis and initiation of appropriate antibiotic therapy, the average case-fatality rate is 20% to 30%.5 Thus, it is important for health care professionals to recognize signs and symptoms early, especially in high-risk populations.
Microbiology
Of the seven existing Listeria species, two—Listeria monocytogenes and Listeria ivanovii—are known to mainly affect humans, and most infections are due to L monocytogenes.6 L monocytogenes is a non–spore-forming, gram-positive bacillus that is cultured in blood agar.7 It is a facultative-anaerobic, catalase-positive bacterium that produces incomplete beta hemolysis when cultured.7 L monocytogenes has a wide incubation time (up to 90 days).8 Additionally, L monocytogenes produces biofilm and is resilient in its ability to survive and replicate in unfavorable conditions; for instance, it can grow at temperatures from –1°C to 45°C, in acidic environments, and in relatively high salt concentrations.5,7 The ubiquitous nature of L monocytogenes and the unique characteristics aforementioned explain why food-source contamination from Listeria species remains a problem.
Under the microscope, L monocytogenes can resemble diphtheroids, potentially leading to misdiagnosis or inconclusive laboratory results. Positive blood culture and cerebrospinal fluid (CSF) laboratory results for diphtheroid should alert the clinician to a possible misdiagnosis. A motility test may be used as a differential; at room temperature, L monocytogenes exhibits a tumbling end-over-end motion.9
Pathogenesis
Listeriosis, which often results from ingestion of contaminated foods containing L monocytogenes, has the ability to cross the intestinal, blood-brain, and fetoplacental barriers. Once it enters the gastrointestinal (GI) tract, the bacterium internalizes within the host’s epithelial cells, where it multiplies and initiates the processes of infection. Internalization of the bacterium can occur by either phagocytosis (in the case of macrophages) or induced phagocytosis (in the case of normally nonphagocytic cells).10,11 Listeria utilizes the cell-wall adhesion proteins internalin A and internalin B to bind to the host-cell membrane receptors E-cadherin and Met. Once the phagocytic vacuole enters the cytoplasm of the cell, a phagolysosome produced by the bacterium, listeriolysin O, lyses the vacuole and allows it to enter the host cytoplasm, where it multiplies.
In the cytoplasm, Listeria spreads cell to cell by actin-based motility.10 Surface proteins on the bacterium, called ActA, initiate actin formation.12 This allows the bacterium to propel to the cell membrane and create filopods. A filopod is an elongated protrusion of the cell membrane that encompasses the bacterial cell and extends to adjacent cells. Adjacent cells ingest the filopods, allowing the bacterium to enter the cytosol of the new cell. This enables Listeria to avoid antibodies, complement, or neutrophils of the host cell. Immunity to Listeria is primarily cell mediated, which explains the greater association between listeriosis and conditions involving impaired cell-mediated immunity, such as pregnancy, AIDS, and organ transplantation.9
Clinical Characteristics
Despite a nationwide reporting and surveillance system, many cases of listeriosis go undiagnosed. This is partly because of the bacterium’s long incubation period and the self-limiting clinical conditions of noninvasive listeriosis.5,8 It may take up to 2 months following host infection for signs of infection to develop, with symptoms typically lasting 2 to 3 days.
Infections can be noninvasive, invasive, or focal. Most cases of listeriosis are noninvasive and present as flulike symptoms (e.g., fever, arthromyalgia). Listeria gastroenteritis (noninvasive) often involves watery, nonbloody diarrhea.13 Other GI complaints include vomiting, nausea, and abdominal pain. Listeriosis can cause an array of focal infections (TABLE 1), although these are rare. Signs and symptoms of focal infections are diverse and highly site specific.
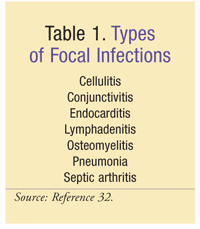
Invasive listeriosis, which can be fatal, occurs when bacteria reach the bloodstream or the central nervous system (CNS). Consequently, this form of listeria can lead to meningitis, brain abscesses, or rhombencephalitis.2 Immunocompromised, very young, and elderly individuals are most susceptible to invasive infection. Symptoms of CNS infection include headache, nuchal rigidity, confusion, loss of balance, and convulsions. In addition to fever, Kernig’s and Brudzinski’s signs may occur in adults with acute meningitis. Although symptoms of acute meningitis are usually less pronounced in children, they commonly include bulging fontanelles, purpuric rash, and seizures; Kernig’s and Brudinski’s signs are often absent.
Pregnant women with invasive listeriosis may be asymptomatic or present with nonspecific flulike symptoms. Primary bacteremia in pregnant women can lead to intrauterine infection, premature labor, stillbirth, and neonatal infections. Neonatal listeriosis is a potentially fatal infection that can cause pneumonia, sepsis, or meningitis. The two forms of neonatal Listeria infection are early-onset syndrome (granulomatosis infantiseptica), occurring hours to days after birth, and late-onset syndrome, occurring more than 5 to 7 days after delivery.4 Common signs and symptoms of neonatal listeriosis are respiratory distress, fever, rash, jaundice, and lethargy.4
Diagnosis
The various clinical manifestations of listeriosis are useful diagnostic guides. Febrile gastroenteritis secondary to Listeria can be difficult to definitively diagnose owing to its general, brief symptom complex resembling other causes of food poisoning. Although recent data list the prevalence of bacterial foodborne infections caused by Listeria species as less than 1% of all reported cases, widespread illness—such as that seen in 2011—can help identify these infections.14 Only positive cultures of the organism from blood or CSF can conclusively diagnose listeriosis infections.15 Gram staining of CSF seems to be much less definitive when Listeria species are the causative pathogens; in fact, L monocytogenes meningitis yields a positive Gram stain in only about 33% of cases.16,17 In addition to low sensitivity, specificity is lacking. A Gram-stained specimen may have a shape more like the diplococcus of pneumococcus, a diphtheroid such as Corynebacteria species, or a Gram-variable organism such as Haemophilus. Therefore, clinicians should hesitate to rule out Listeria based solely on a seemingly negative or inconclusive Gram stain.18
Treatment
Treatment recommendations are made secondary to the location, severity, and extent of infection. Most immunocompetent patients who develop febrile gastroenteritis after consuming Listeria-contaminated foods have symptom resolution within 2 days, and usually prior to identification of the offending organism.13 For this reason, these patients rarely require or receive antimicrobial therapy. The progression from gastroenteritis to more invasive disease is relatively uncommon. However, the likelihood increases in pregnant, immunocompromised, and elderly patients.3,19 To help prevent progression to more serious forms of listeriosis in these populations, treatment with oral ampicillin or sulfamethoxazole-trimethoprim (SMX-TMP) has been suggested.13
In patients with severe infection (e.g., CNS infection, bacteremia, endocarditis), treatment is vitally important. The first-line drugs of choice for the treatment of severe listeriosis are ampicillin and penicillin G.15,20 General dosing of these agents varies based on patient age and weight (TABLE 2). Regardless of CNS involvement, meningitis dosing should be implemented secondary to the inclination of this organism toward the CNS.15 However, a limitation is that both of these agents—at the concentrations generally achieved in CSF—show delayed bactericidal activity in vitro.15,21 Because of the possibility of progression to and within the CNS with this delayed bactericidal activity, it is imperative to employ additional therapy. Multiple references have documented the synergistic benefit of adding gentamicin to the regimen in higher-risk patients (i.e., immunocompromised, pregnant, neonatal) with listerial CNS infections and endocarditis.20,22,23 Gentamicin dosing for synergy should be patient-specific and based on serum peaks and troughs, with a goal peak of 3 to 5 mcg/mL and a trough less than 1 mcg/mL.16,24,25
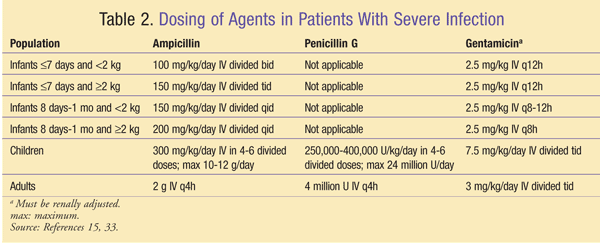
Practice guidelines for the management of bacterial meningitis provide detailed recommendations for the treatment of L monocytogenes infection in the CNS.16 Patients aged less than 1 month, those aged greater than 50 years, alcoholic patients, and immunocompromised patients are at increased risk for Listeria infections in the CNS.16 Patients with HIV or AIDS contract Listeria meningitis at a rate exceeding more than 60 times that of the general population.26 These populations should be empirically treated for coverage of L monocytogenes. Neonates aged less than 1 month should receive ampicillin plus ceftriaxone or ampicillin plus an aminoglycoside. Elderly patients, alcoholic patients, and those with compromised immune systems should be empirically treated with a three-drug regimen of vancomycin, ampicillin, and a third-generation cephalosporin with or without dexamethasone. Therapy may be de-escalated to ampicillin or penicillin G alone upon confirmation of L monocytogenes. Alternatives include SMX-TMP and meropenem.16 Antimicrobial therapy for meningitis should last at least 21 days, with longer therapy (≥8 weeks) favored in cases of rhombencephalitis, brain abscess, and compromised immunity.15,16,27,28
Several potential medication-related issues may arise in the treatment of a patient with listeriosis. Patients allergic to penicillin may need to undergo skin testing and subsequent desensitization protocols. Individuals whose allergy is limited to mild-to-moderate maculopapular rash may use meropenem (adults, 2 g IV q8h; children, 120 mg/kg/day IV divided over 3 daily doses).29 If the patient has a severe penicillin allergy (e.g., type I, immunoglobulin E [IgE]–mediated allergy, Stevens-Johnson syndrome [SJS] or toxic epidermal necrolysis), SMX-TMP 10 mg to 20 mg trimethoprim/kg/day IV divided every 6 to 12 hours may be utilized, with the higher end of the dosing spectrum favored for more severe conditions. When feasible, patients taking IV SMX-TMP can be converted to oral therapy at the same dosage.30
Another potential treatment issue occurs in pregnant women. Invasive disease can be treated as outlined above, with ampicillin alone as the drug of choice with desensitization in non–IgE-mediated, non-SJS, penicillin-allergic patients, or with SMX-TMP in patients with more severe allergy. However, SMX-TMP can cause potentially serious adverse effects in pregnant women. Because of disturbances in folic acid metabolism, SMX-TMP should be used only in the first trimester when the potential benefits to the mother outweigh the risks of fetal harm. Also, the use of SMX-TMP late in pregnancy may cause kernicterus in the child.30 Alternatively, vancomycin may be used in patients who cannot tolerate desensitization and in those cannot receive SMX-TMP based on pregnancy trimester.15
Clinicians treating organ-transplant recipients who have contracted listeriosis must be aware of the potential drug interactions and complications. These patients may be taking tacrolimus or cyclosporine, so it is important to closely monitor renal function should SMX-TMP or synergistic treatment with gentamicin be necessary, as the combination of these agents can further increase the risk of nephrotoxicity. These patients may also require reduced intensity in their immunosuppressive regimens if controlling infection becomes difficult. The decision should be individualized, taking into consideration not only benefits, but also the risk of acute organ rejection.
Prevention
Since listeriosis is most often associated with contaminated food, several preventive steps can be taken in food storage and preparation to avoid the development of this potentially serious infection.15,31
• Thoroughly rinse or wash raw produce, even if peeled.
• Fully cook raw meats, poultry, and animal products to a safe and appropriate internal temperature.
• Keep raw produce, meats, and poultry separate from other ingredients, cooked foods, and ready-to-eat foods.
• Frequently wash the hands while handling raw food products; wash knives, countertops, and cutting boards after preparing raw food products.
• Because Listeria species can grow at refrigeration temperatures, it is important to keep refrigerators clean with hot water and liquid soap.
• Do not drink or eat foods containing unpasteurized milk.
Conclusion
Listeriosis, a serious and potentially fatal infection, typically results from foodborne contamination. Although most cases of infection are self-limiting, it is critically important to utilize appropriate antibiotic therapy in patients at high risk for developing invasive listeriosis. There are a number of preventive steps related to food storage and preparation that one can take to avoid developing listeriosis.
REFERENCES
1. CDC. Listeria (listeriosis). People at risk. www.cdc.gov/Listeria/risk.html. Accessed March 24, 2012.
2. Todd EC, Notermans S. Surveillance of listeriosis and its causative pathogen, Listeria monocytogenes. Food Control. 2011;22:1484-1490.
3. Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe—United States, August–September 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1357-1358.
4. Janakiraman V. Listeriosis in pregnancy: diagnosis, treatment, and prevention. Rev Obstet Gynecol. 2008;1:179-185.
5. Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect. 2007;9:1236-1243.
6. Guillet C, Join-Lambert O, Le Monnier A, et al. Human listeriosis caused by Listeria ivanovii. Emerg Infect Dis. 2010;16:136-138.
7. Wing EJ, Gregory SH. Listeria monocytogenes: clinical and experimental update. J Infect Dis. 2002;185(suppl 1):S18-S24.
8. FDA. Food. Research areas. www.fda.gov/Food/ScienceResearch/ResearchAreas. Accessed June 26, 2012.
9. Morse SA, Brooks GF, Carroll KC, et al. Aerobic nonspore-forming gram-positive bacilli: Corynebacterium, Listeria, Erysipelothrix, Actinomycetes, & related pathogens. In: Brooks GF, Carroll KC, Butel JS, et al, eds. Jawetz, Melnick, & Adelberg’s Medical Microbiology. 25th ed. New York, NY: McGraw-Hill; 2010.
10. Cossart P, Pizarro-Cerdá J, Lecuit M. Invasion of mammalian cells by Listeria monocytogenes: functional mimicry to subvert cellular functions. Trends Cell Biol. 2003;13:23-31.
11. Cossart P, Lecuit M. Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. EMBO J. 1998;17:3797-3806.
12. Seveau S, Pizarro-Cerdá J, Cossart P. Molecular mechanisms exploited by Listeria monocytogenes during host cell invasion. Microbes Infect. 2007;9:1167-1175.
13. Ooi ST, Lorber B. Gastroenteritis due to Listeria monocytogenes. Clin Infect Dis. 2005;40:1327-1332.
14. Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7-15.
15. Lorber B. Listeria monocytogenes. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010:2707-2713.
16. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.
17. Brouwer MC, van de Beek D, Heckenberg SG, et al. Community-acquired Listeria monocytogenes meningitis in adults. Clin Infect Dis. 2006;43:1233-1238.
18. Gray ML, Killinger AH. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966;30:309-382.
19. Büla CJ, Bille J, Glauser MP. An epidemic of food-borne
listeriosis in western Switzerland: description of 57 cases involving
adults. Clin Infect Dis. 1995;20:66-72.
20. Mylonakis E, Hohmann EL, Calderwood SB. Central nervous system infection with Listeria monocytogenes. 33 years’ experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore). 1998;77:313-336.
21. Lorber B. Listeriosis. Clin Infect Dis. 1997;24:1-11.
22. Drevets DA, Canono BP, Leenen PJ, Campbell PA. Gentamicin kills intracellular Listeria monocytogenes. Infect Immun. 1994;62:2222-2228.
23. Hof H, Nichterlein T, Kretschmar M. Management of listeriosis. Clin Microbiol Rev. 1997;10:345-357.
24. Lexi-Comp Online. Gentamicin (systemic). Hudson, OH: Lexi-Comp, Inc; 2012 [by subscription].
25. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis:
diagnosis, antimicrobial therapy, and management of complications: a
statement for healthcare professionals from the Committee on Rheumatic
Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular
Disease in the Young, and the Councils on Clinical Cardiology, Stroke,
and Cardiovascular Surgery and Anesthesia, American Heart Association:
endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394-e434.
26. Jurardo RL, Farley MM, Pereira E, et al. Increased risk of meningitis and bacteremia due to Listeria monocytogenes in patients with human immunodeficiency virus infection. Clin Infect Dis.1993;17:224-227.
27. Gilbert DN, Moellering RC Jr, Eliopoulos GM, et al, eds. The Sanford Guide to Antimicrobial Therapy 2011. 41st ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2011:66.
28. Armstrong RW, Fung PC. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clin Infect Dis. 1993;16:689-702.
29. Lexi-Comp Online. Meropenem. Hudson, OH: Lexi-Comp, Inc; 2012 [by subscription].
30. Lexi-Comp Online. Sulfamethoxazole and trimethoprim. Hudson, OH: Lexi-Comp, Inc; 2012 [by subscription].
31. CDC. Listeria (listeriosis). Prevention. www.cdc.gov/listeria/prevention.html. Accessed May 3, 2012.
32. Bartlett JG. Listeria monocytogenes. Johns Hopkins ABX Guide.
www.hopkinsguides.com/hopkins/ub/view/Johns_Hopkins_ABX_Guide/540318/all/Listeria_Monocytogenes.
Accessed March 24, 2012.
33. Bortolussi R, Mailman TL. Listeriosis. In: Remington JS, Klein JO, Wilson CB, et al, eds. Infectious Diseases of the Fetus and Newborn Infant. 7th ed. Philadelphia, PA: Elsevier Saunders; 2011:470.
To comment on this article, contact rdavidson@uspharmacist.com.





